Lab Med Online.
2016 Oct;6(4):214-220. 10.3343/lmo.2016.6.4.214.
Monitoring the Antiplatelet Effect of Cilostazol with Light Transmission Aggregometer: Two Cases of Possible Cilostazol Resistance
- Affiliations
-
- 1Seegene Medical Foundation, Seoul, Korea.
- 2Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea. ssjang@amc.seoul.kr
- 3Department of Cardiology, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea.
- KMID: 2353422
- DOI: http://doi.org/10.3343/lmo.2016.6.4.214
Abstract
- BACKGROUND
Coronary artery disease is an important cause of death in adults and stent insertion is one of the treatment modalities. The most severe adverse effect of a stent insertion is the formation of a thrombus; therefore, antiplatelet agents are used. The addition of cilostazol to low-dose aspirin and clopidogrel results in a better antiplatelet effect. However, laboratory tests to monitor the effect of cilostazol are insufficient.
METHODS
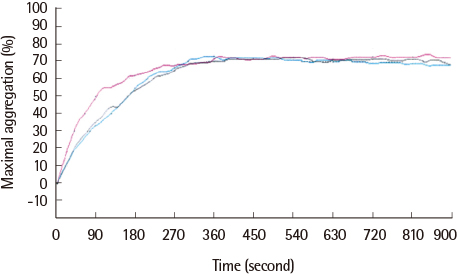
We tested the inhibitory effect of cilostazol using maximal platelet aggregation in 20 healthy volunteers. Conditions for incubation and concentrations of cilostazol and prostaglandin E1 (PGE1) were established and aggregation was induced by 5'-adenosine diphosphate (ADP) and measured with light transmission aggregometry (LTA). Blood samples were incubated with 1 µM and 2 µM cilostazol for 10 minutes at room temperature, and 80 nM PGE1 was added and incubated for an additional 10 minutes. Aggregation was induced by ADP and reactivity was evaluated.
RESULTS
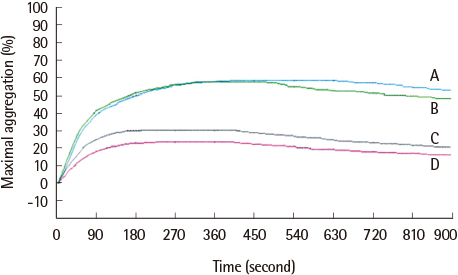
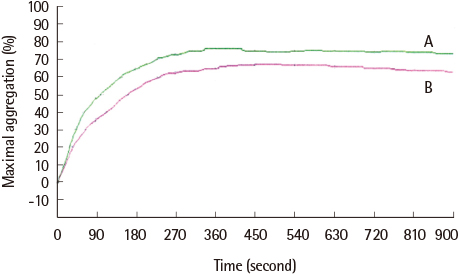
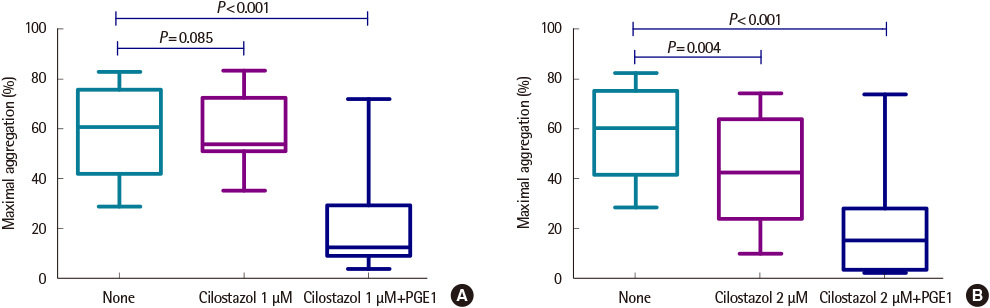
The average maximum aggregation (MA) was 58.1% at 1 µM cilostazol and 22.0% when PGE1 was added. The average MA was 42.8% when cilostazol concentration was increased to 2 µM and 21.2% when PGE1 was added. Average inhibition of aggregation at 1 µM cilostazol was not statistically significant (P=0.085), but was significant (P=0.004) at 2 µM cilostazol. Aggregation was not inhibited even with 2 µM cilostazol and PGE1 in 2 volunteers, which suggests possible resistance to cilostazol.
CONCLUSIONS
We designed a method to monitor the effect of cilostazol using in vitro incubation with PGE1.
MeSH Terms
Figure
Reference
-
1. Sudo T, Ito H, Ozeki Y, Kimura Y. Estimation of anti-platelet drugs on human platelet aggregation with a novel whole blood aggregometer by a screen filtration pressure method. Br J Pharmacol. 2001; 133:1396–1404.
Article2. Gum PA, Kottke-Marchant K, Poggio ED, Gurm H, Welsh PA, Brooks L, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001; 88:230–235.
Article3. Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003; 107:2908–2913.4. Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, et al. Comparison of triple versus dual antiplatelet therapy after drug-eluting stent implantation (from the DECLARE-Long trial). Am J Cardiol. 2007; 100:1103–1108.
Article5. Yamamoto Y, Yasuda Y, Kimura Y, Komiya Y. Effects of cilostazol, an antiplatelet agent, on axonal regeneration following nerve injury in diabetic rats. Eur J Pharmacol. 1998; 352:171–178.
Article6. Yamamoto H, Takahashi K, Watanabe H, Yoshikawa Y, Shirakawa R, Higashi T, et al. Evaluation of the antiplatelet effects of cilostazol, a phosphodiesterase 3 inhibitor, by VASP phosphorylation and platelet aggregation. Circ J. 2008; 72:1844–1851.
Article7. Russo I, Doronzo G, Mattiello L, De Salve A, Trovati M, Anfossi G. The activity of constitutive nitric oxide synthase is increased by the pathway cAMP/cAMP-activated protein kinase in human platelets. New insights into the antiaggregating effects of cAMP-elevating agents. Thromb Res. 2004; 114:265–273.
Article8. Bramer SL, Forbes WP, Mallikaarjun S. Cilostazol pharmacokinetics after single and multiple oral doses in healthy males and patients with intermittent claudication resulting from peripheral arterial disease. Clin Pharmacokinet. 1999; 37:Suppl 2. 1–11.
Article9. Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tour-nier B, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001; 345:1809–1817.
Article10. Csiszar A, Stef G, Pacher P, Ungvari Z. Oxidative stress-induced isoprostane formation may contribute to aspirin resistance in platelets. Prostaglandins Leukot Essent Fatty Acids. 2002; 66:557–558.
Article11. Szczeklik A, Musial J, Undas A, Swadzba J, Gora PF, Piwowarska W, et al. Inhibition of thrombin generation by aspirin is blunted in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1996; 16:948–954.
Article12. Christiaens L, Macchi L, Herpin D, Coisne D, Duplantier C, Allal J, et al. Resistance to aspirin in vitro at rest and during exercise in patients with angiographically proven coronary artery disease. Thromb Res. 2002; 108:115–119.
Article13. Goto S. Antiplatelet therapy after coronary intervention in Asia and Japan: the Asian perspective of antiplatelet intervention. Hamostaseologie. 2009; 29:321–325.14. Uchiyama S, Fukuuchi Y, Yamaguchi T. The safety and efficacy of clopidogrel versus ticlopidine in Japanese stroke patients: combined results of two Phase III, multicenter, randomized clinical trials. J Neurol. 2009; 256:888–897.
Article15. Chan FK, Ching JY, Hung LC, Wong VW, Leung VK, Kung NN, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med. 2005; 352:238–244.
Article16. Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008; 52:1502–1517.17. Yamamoto T, Ebato T, Mishina Y, Abe K, Hattori K, Ishii T, et al. Thienopyridine and cilostazol are safer for gastroduodenal mucosa than low-dose aspirin--second report of endoscopic evaluation. Thromb Res. 2010; 125:365–366.
Article18. Jeong YH, Lee SW, Choi BR, Kim IS, Seo MK, Kwak CH, et al. Rando-mized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity: results of the ACCEL-RESISTANCE (Adjunctive Cilostazol Versus High Maintenance Dose Clopidogrel in Patients With Clopidogrel Resistance) randomized study. J Am Coll Cardiol. 2009; 53:1101–1109.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Study of Ex Vivo Antiplatelet Activity of Aspirin and Cilostazol in Patients with Diabetes and High Risk of Cardiovascular Disease
- Spontaneous Spinal Epidural Hematoma in a Patient on Cilostazol
- Can Cilostazol Improve the Patency Rate of Native Arteriovenous Fistula in Hemodialysis Patients?
- The Effect of Cilostazol on Stent Thrombosis After Drug-Eluting Stent Implantation
- Protective Effect of Cilostazol Against Restraint Stress Induced Heart Failure in Post-Myocardial Infarction Rat Model