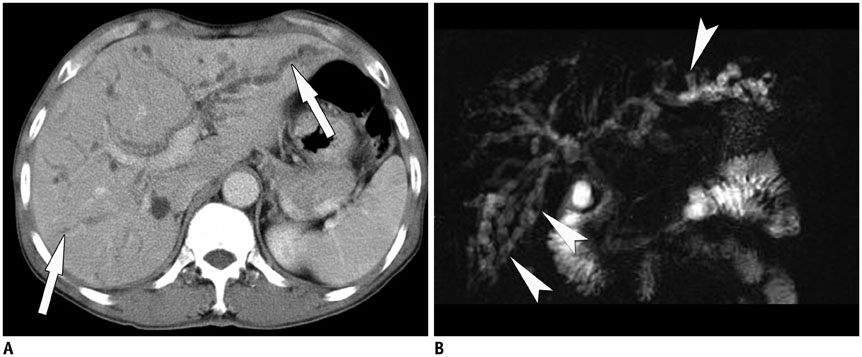
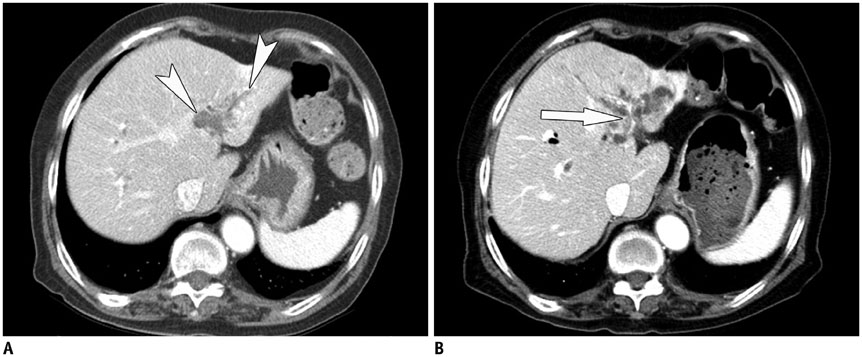
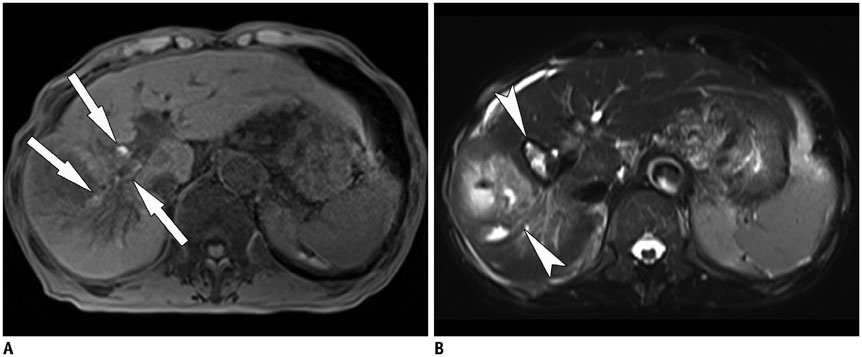
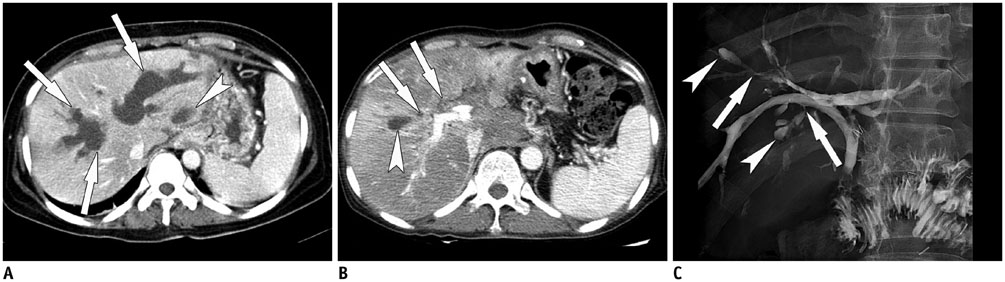
Korean J Radiol.
2016 Feb;17(1):25-38. 10.3348/kjr.2016.17.1.25.
Sclerosing Cholangitis: Clinicopathologic Features, Imaging Spectrum, and Systemic Approach to Differential Diagnosis
- Affiliations
-
- 1Department of Radiology, Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Korea.
- 2Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea. sykimrad@amc.seoul.kr
- KMID: 2351160
- DOI: http://doi.org/10.3348/kjr.2016.17.1.25
Abstract
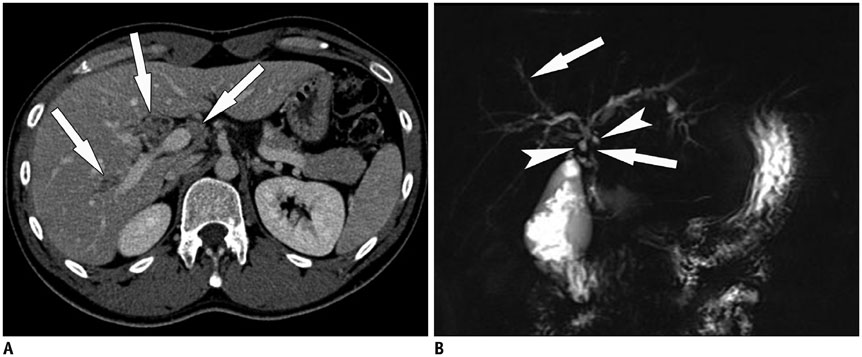
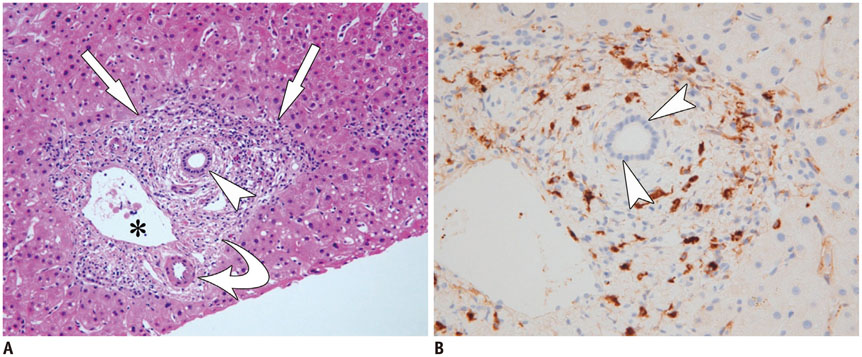
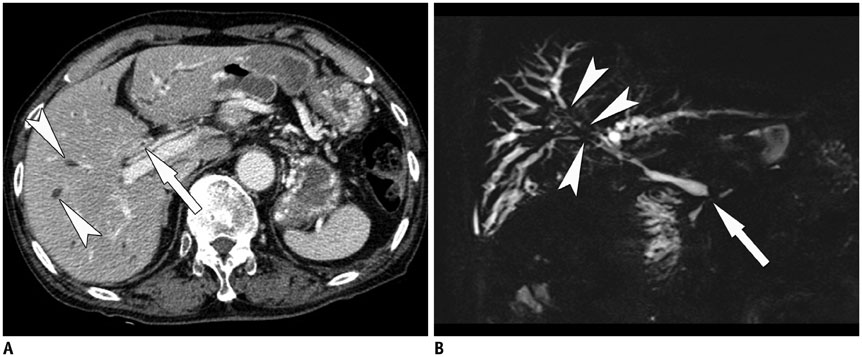
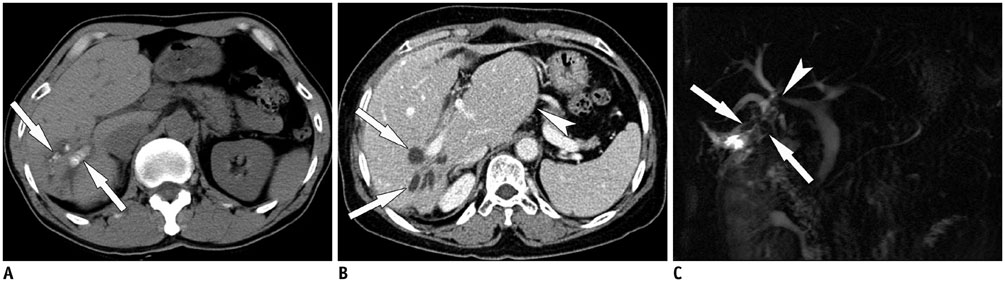
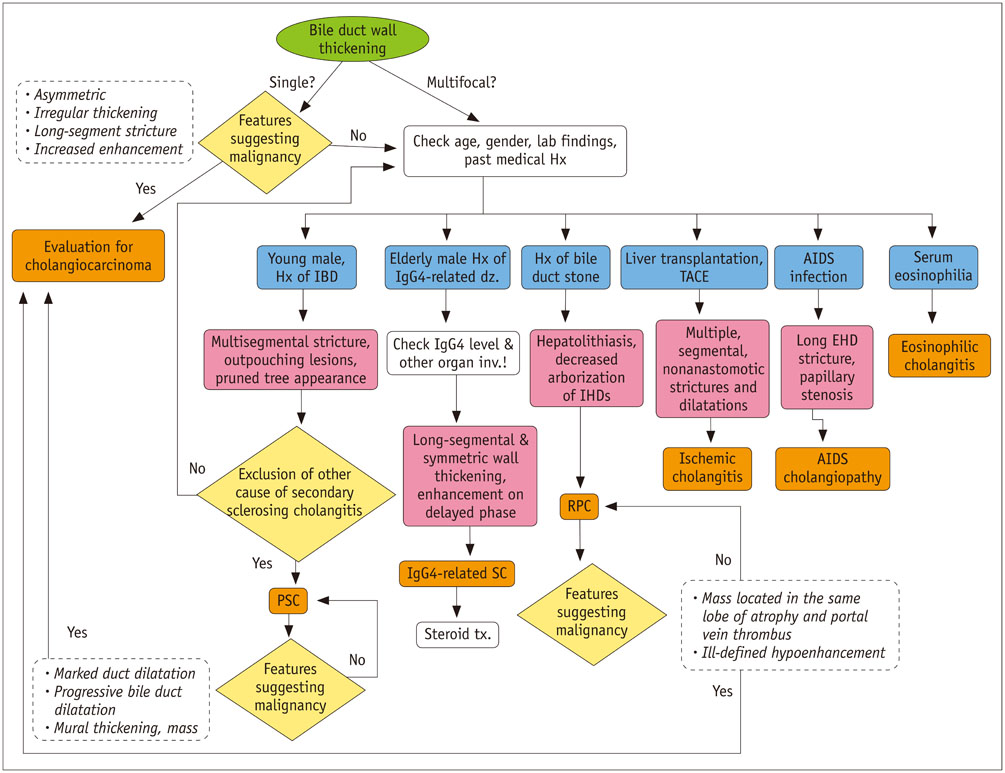
- Sclerosing cholangitis is a spectrum of chronic progressive cholestatic liver disease characterized by inflammation, fibrosis, and stricture of the bile ducts, which can be classified as primary and secondary sclerosing cholangitis. Primary sclerosing cholangitis is a chronic progressive liver disease of unknown cause. On the other hand, secondary sclerosing cholangitis has identifiable causes that include immunoglobulin G4-related sclerosing disease, recurrent pyogenic cholangitis, ischemic cholangitis, acquired immunodeficiency syndrome-related cholangitis, and eosinophilic cholangitis. In this review, we suggest a systemic approach to the differential diagnosis of sclerosing cholangitis based on the clinical and laboratory findings, as well as the typical imaging features on computed tomography and magnetic resonance (MR) imaging with MR cholangiography. Familiarity with various etiologies of sclerosing cholangitis and awareness of their typical clinical and imaging findings are essential for an accurate diagnosis and appropriate management.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Bile Ducts/*pathology
Cholangiography/*methods
Cholangitis/diagnosis/*pathology
Cholangitis, Sclerosing/*diagnosis/pathology
Cholestasis/diagnosis/*pathology
Chronic Disease
Constriction, Pathologic/diagnosis
Diagnosis, Differential
Female
Humans
Immunoglobulin G/immunology
Liver/pathology
Magnetic Resonance Imaging/methods
Male
Middle Aged
Tomography, X-Ray Computed/methods
Immunoglobulin G
Figure
Reference
-
1. Azizi L, Raynal M, Cazejust J, Ruiz A, Menu Y, Arrivé L. MR Imaging of sclerosing cholangitis. Clin Res Hepatol Gastroenterol. 2012; 36:130–138.2. Maggs JR, Chapman RW. An update on primary sclerosing cholangitis. Curr Opin Gastroenterol. 2008; 24:377–383.3. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007; 102:107–114.4. Kingham JG, Kochar N, Gravenor MB. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology. 2004; 126:1929–1930.5. Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003; 125:1364–1369.6. Escorsell A, Parés A, Rodés J, Solís-Herruzo JA, Miras M, de la Morena E. Spanish Association for the Study of the Liver. Epidemiology of primary sclerosing cholangitis in Spain. J Hepatol. 1994; 21:787–791.7. Ang TL, Fock KM, Ng TM, Teo EK, Chua TS, Tan JY. Clinical profile of primary sclerosing cholangitis in Singapore. J Gastroenterol Hepatol. 2002; 17:908–913.8. Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980; 79:200–206.9. Elsayes KM, Oliveira EP, Narra VR, Abou El Abbass HA, Ahmed MI, Tongdee R, et al. MR and MRCP in the evaluation of primary sclerosing cholangitis: current applications and imaging findings. J Comput Assist Tomogr. 2006; 30:398–404.10. Saarinen S, Olerup O, Broomé U. Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2000; 95:3195–3199.11. Chapman RW, Jewell DP. Primary sclerosing cholangitis--an immunologically mediated disease? West J Med. 1985; 143:193–195.12. Karlsen TH, Franke A, Melum E, Kaser A, Hov JR, Balschun T, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010; 138:1102–1111.13. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013; 382:1587–1599.14. Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010; 51:660–678.15. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009; 51:237–267.16. Vitellas KM, Keogan MT, Freed KS, Enns RA, Spritzer CE, Baillie JM, et al. Radiologic manifestations of sclerosing cholangitis with emphasis on MR cholangiopancreatography. Radiographics. 2000; 20:959–975. quiz 1108-1109, 111217. Gulliver DJ, Baker ME, Putnam W, Baillie J, Rice R, Cotton PB. Bile duct diverticula and webs: nonspecific cholangiographic features of primary sclerosing cholangitis. AJR Am J Roentgenol. 1991; 157:281–285.18. Fulcher AS, Turner MA, Franklin KJ, Shiffman ML, Sterling RK, Luketic VA, et al. Primary sclerosing cholangitis: evaluation with MR cholangiography-a case-control study. Radiology. 2000; 215:71–80.19. Ament AE, Haaga JR, Wiedenmann SD, Barkmeier JD, Morrison SC. Primary sclerosing cholangitis: CT findings. J Comput Assist Tomogr. 1983; 7:795–800.20. Revelon G, Rashid A, Kawamoto S, Bluemke DA. Primary sclerosing cholangitis: MR imaging findings with pathologic correlation. AJR Am J Roentgenol. 1999; 173:1037–1042.21. Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009; 50:158–164.22. Cullen SN, Chapman RW. The medical management of primary sclerosing cholangitis. Semin Liver Dis. 2006; 26:52–61.23. Campsen J, Zimmerman MA, Trotter JF, Wachs M, Bak T, Steinberg T, et al. Clinically recurrent primary sclerosing cholangitis following liver transplantation: a time course. Liver Transpl. 2008; 14:181–185.24. Stone JH. IgG4-related disease: nomenclature, clinical features, and treatment. Semin Diagn Pathol. 2012; 29:177–190.25. Nishino T, Oyama H, Hashimoto E, Toki F, Oi I, Kobayashi M, et al. Clinicopathological differentiation between sclerosing cholangitis with autoimmune pancreatitis and primary sclerosing cholangitis. J Gastroenterol. 2007; 42:550–559.26. Zen Y, Nakanuma Y, Portmann B. Immunoglobulin G4-related sclerosing cholangitis: pathologic features and histologic mimics. Semin Diagn Pathol. 2012; 29:205–211.27. Vlachou PA, Khalili K, Jang HJ, Fischer S, Hirschfield GM, Kim TK. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. 2011; 31:1379–1402.28. Itoh S, Nagasaka T, Suzuki K, Satake H, Ota T, Naganawa S. Lymphoplasmacytic sclerosing cholangitis: assessment of clinical, CT, and pathological findings. Clin Radiol. 2009; 64:1104–1114.29. Kawamoto S, Siegelman SS, Hruban RH, Fishman EK. Lymphoplasmacytic sclerosing pancreatitis (autoimmune pancreatitis): evaluation with multidetector CT. Radiographics. 2008; 28:157–170.30. Nakazawa T, Ohara H, Sano H, Aoki S, Kobayashi S, Okamoto T, et al. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointest Endosc. 2004; 60:937–944.31. Nishino T, Toki F, Oyama H, Oi I, Kobayashi M, Takasaki K, et al. Biliary tract involvement in autoimmune pancreatitis. Pancreas. 2005; 30:76–82.32. Kim JH, Byun JH, Lee SJ, Park SH, Kim HJ, Lee SS, et al. Differential diagnosis of sclerosing cholangitis with autoimmune pancreatitis and periductal infiltrating cancer in the common bile duct at dynamic CT, endoscopic retrograde cholangiography and MR cholangiography. Eur Radiol. 2012; 22:2502–2513.33. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011; 40:352–358.34. Catalano OA, Sahani DV, Forcione DG, Czermak B, Liu CH, Soricelli A, et al. Biliary infections: spectrum of imaging findings and management. Radiographics. 2009; 29:2059–2080.35. Al-Sukhni W, Gallinger S, Pratzer A, Wei A, Ho CS, Kortan P, et al. Recurrent pyogenic cholangitis with hepatolithiasis--the role of surgical therapy in North America. J Gastrointest Surg. 2008; 12:496–503.36. Okuno WT, Whitman GJ, Chew FS. Recurrent pyogenic cholangiohepatitis. AJR Am J Roentgenol. 1996; 167:484.37. Kim MH, Sekijima J, Lee SP. Primary intrahepatic stones. Am J Gastroenterol. 1995; 90:540–548.38. Tsui WM, Chan YK, Wong CT, Lo YF, Yeung YW, Lee YW. Hepatolithiasis and the syndrome of recurrent pyogenic cholangitis: clinical, radiologic, and pathologic features. Semin Liver Dis. 2011; 31:33–48.39. Leung JW, Yu AS. Hepatolithiasis and biliary parasites. Baillieres Clin Gastroenterol. 1997; 11:681–706.40. Heffernan EJ, Geoghegan T, Munk PL, Ho SG, Harris AC. Recurrent pyogenic cholangitis: from imaging to intervention. AJR Am J Roentgenol. 2009; 192:W28–W35.41. Afagh A, Pancu D. Radiologic findings in recurrent pyogenic cholangitis. J Emerg Med. 2004; 26:343–346.42. Chan FL, Man SW, Leong LL, Fan ST. Evaluation of recurrent pyogenic cholangitis with CT: analysis of 50 patients. Radiology. 1989; 170(1 Pt 1):165–169.43. Kim MJ, Cha SW, Mitchell DG, Chung JJ, Park S, Chung JB. MR imaging findings in recurrent pyogenic cholangitis. AJR Am J Roentgenol. 1999; 173:1545–1549.44. Yeh BM, Liu PS, Soto JA, Corvera CA, Hussain HK. MR imaging and CT of the biliary tract. Radiographics. 2009; 29:1669–1688.45. Lim JH, Mairiang E, Ahn GH. Biliary parasitic diseases including clonorchiasis, opisthorchiasis and fascioliasis. Abdom Imaging. 2008; 33:157–165.46. Menias CO, Surabhi VR, Prasad SR, Wang HL, Narra VR, Chintapalli KN. Mimics of cholangiocarcinoma: spectrum of disease. Radiographics. 2008; 28:1115–1129.47. Kim JH, Kim TK, Eun HW, Byun JY, Lee MG, Ha HK, et al. CT findings of cholangiocarcinoma associated with recurrent pyogenic cholangitis. AJR Am J Roentgenol. 2006; 187:1571–1577.48. Park MS, Yu JS, Kim KW, Kim MJ, Chung JP, Yoon SW, et al. Recurrent pyogenic cholangitis: comparison between MR cholangiography and direct cholangiography. Radiology. 2001; 220:677–682.49. Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979; 66:379–384.50. Deltenre P, Valla DC. Ischemic cholangiopathy. J Hepatol. 2006; 44:806–817.51. Deltenre P, Valla DC. Ischemic cholangiopathy. Semin Liver Dis. 2008; 28:235–246.52. Kinner S, Umutlu L, Dechêne A, Ladd SC, Barkhausen J, Gerken G, et al. Biliary complications after liver transplantation: addition of T1-weighted images to MR cholangiopancreatography facilitates detection of cast in biliary cast syndrome. Radiology. 2012; 263:429–436.53. Valente JF, Alonso MH, Weber FL, Hanto DW. Late hepatic artery thrombosis in liver allograft recipients is associated with intrahepatic biliary necrosis. Transplantation. 1996; 61:61–65.54. Keaveny AP, Karasik MS. Hepatobiliary and pancreatic infections in AIDS: Part one. AIDS Patient Care STDS. 1998; 12:347–357.55. Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006; 44:1063–1074.56. Keaveny AP, Karasik MS. Hepatobiliary and pancreatic infections in AIDS: Part II. AIDS Patient Care STDS. 1998; 12:451–456.57. Mahajani RV, Uzer MF. Cholangiopathy in HIV-infected patients. Clin Liver Dis. 1999; 3:669–684. x58. Cello JP, Chan MF. Long-term follow-up of endoscopic retrograde cholangiopancreatography sphincterotomy for patients with acquired immune deficiency syndrome papillary stenosis. Am J Med. 1995; 99:600–603.59. Bilgin M, Balci NC, Erdogan A, Momtahen AJ, Alkaade S, Rau WS. Hepatobiliary and pancreatic MRI and MRCP findings in patients with HIV infection. AJR Am J Roentgenol. 2008; 191:228–232.60. Bader TR, Braga L, Beavers KL, Semelka RC. MR imaging findings of infectious cholangitis. Magn Reson Imaging. 2001; 19:781–788.61. Wilcox CM, Mönkemüller KE. Hepatobiliary diseases in patients with AIDS: focus on AIDS cholangiopathy and gallbladder disease. Dig Dis. 1998; 16:205–213.62. Forbes A, Blanshard C, Gazzard B. Natural history of AIDS related sclerosing cholangitis: a study of 20 cases. Gut. 1993; 34:116–121.63. Cordero E, López-Cortés LF, Belda O, Villanueva JL, Rodríguez-Hernández MJ, Pachn J. Acquired immunodeficiency syndrome-related cryptosporidial cholangitis: resolution with endobiliary prosthesis insertion. Gastrointest Endosc. 2001; 53:534–535.64. Vauthey JN, Loyer E, Chokshi P, Lahoti S. Case 57: eosinophilic cholangiopathy. Radiology. 2003; 227:107–112.65. Miura F, Asano T, Amano H, Yoshida M, Toyota N, Wada K, et al. Resected case of eosinophilic cholangiopathy presenting with secondary sclerosing cholangitis. World J Gastroenterol. 2009; 15:1394–1397.66. Butler TW, Feintuch TA, Caine WP Jr. Eosinophilic cholangitis, lymphadenopathy, and peripheral eosinophilia: a case report. Am J Gastroenterol. 1985; 80:572–574.67. Nashed C, Sakpal SV, Shusharina V, Chamberlain RS. Eosinophilic cholangitis and cholangiopathy: a sheep in wolves clothing. HPB Surg. 2010; 2010:906496.68. Rosengart TK, Rotterdam H, Ranson JH. Eosinophilic cholangitis: a self-limited cause of extrahepatic biliary obstruction. Am J Gastroenterol. 1990; 85:582–585.69. Song HH, Byun JY, Jung SE, Choi KH, Shinn KS, Kim BK. Eosinophilic cholangitis: US, CT, and cholangiography findings. J Comput Assist Tomogr. 1997; 21:251–253.70. Kim JY, Lee JM, Han JK, Kim SH, Lee JY, Choi JY, et al. Contrast-enhanced MRI combined with MR cholangiopancreatography for the evaluation of patients with biliary strictures: differentiation of malignant from benign bile duct strictures. J Magn Reson Imaging. 2007; 26:304–312.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunoglobulin G4-related sclerosing cholangitis
- Primary Sclerosing Cholangitis
- Primary Sclerosing Cholangitis: One Case Report
- Biliary Tract & Pancreas; A Case of Cholangiocarcinoma Suggested as Developing in the Patient with Primary Sclerosing Cholangitis
- Comparison of Clinical Findings between Autoimmune Pancreatitis with Bile Duct Involvement and Primary Sclerosing Cholangitis