J Korean Med Sci.
2015 Nov;30(11):1577-1583. 10.3346/jkms.2015.30.11.1577.
Response-Guided Therapy for Hepatitis C Virus Recurrence Based on Early Protocol Biopsy after Liver Transplantation
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. kwleegs@gmail.com
- KMID: 2351106
- DOI: http://doi.org/10.3346/jkms.2015.30.11.1577
Abstract
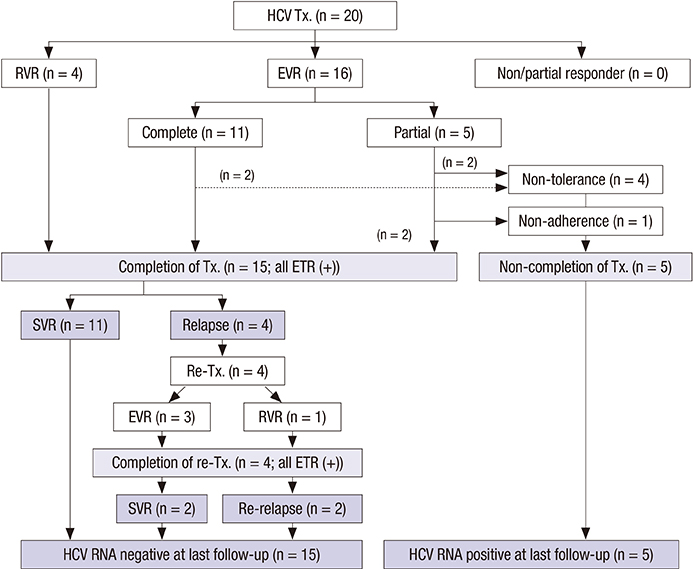
- Hepatitis C virus (HCV) recurrence after liver transplantation (LT) is universal and progressive. Here, we report recent results of response-guided therapy for HCV recurrence based on early protocol biopsy after LT. We reviewed patients who underwent LT for HCV related liver disease between 2010 and 2012. Protocol biopsies were performed at 3, 6, and 12 months after LT in HCV recurrence (positive HCV-RNA). For any degree of fibrosis, > or = moderate inflammation on histology or HCV hepatitis accompanying with abnormal liver function, we treated with pegylated interferon and ribavirin. We adjusted treatment period according to individual response to treatment. Among 41 HCV related recipients, 25 (61.0%) who underwent protocol biopsies more than once were enrolled in this study. The mean follow-up time was 43.1 (range, 23-55) months after LT. Genotype 1 and 2 showed in 56.0% and 36.0% patients, respectively. Of the 25 patients, 20 (80.0%) started HCV treatment after LT. Rapid or early virological response was observed in 20 (100%) patients. Fifteen (75.0%) patients finished the treatment with end-of-treatment response. Sustained virological response (SVR) was in 11 (55.0%) patients, including 5 (41.7%) of 12 genotype 1 and 6 (75.0%) of 8 non-genotype 1 (P = 0.197). Only rapid or complete early virological response was a significant predictor for HCV treatment response after LT (100% in SVR group vs. 55.6% in non-SVR group, P = 0.026). Overall 3-yr survival rate was 100%. In conclusion, response-guided therapy for HCV recurrence based on early protocol biopsy after LT shows encouraging results.
Keyword
MeSH Terms
-
Adult
Aged
Antiviral Agents/*administration & dosage
Biopsy
Drug Monitoring/*methods
Female
Hepatitis C/etiology/*pathology/*prevention & control
Humans
Liver Transplantation/*adverse effects
Male
Middle Aged
Recurrence
Reproducibility of Results
Retrospective Studies
Sensitivity and Specificity
Treatment Outcome
Watchful Waiting/methods
Antiviral Agents
Figure
Reference
-
1. Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, Maertens G, Williams R. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996; 334:815–820.2. Agarwal K, Barnabas A. Treatment of chronic hepatitis C virus infection after liver transplantation. Dig Liver Dis. 2013; 45:S349–S354.3. Lange CM. The importance of IL28B genotype in hepatitis C virus-associated liver transplantation. Liver Int. 2013; 33:169–171.4. Rubin A, Aguilera V, Berenguer M. Liver transplantation and hepatitis C. Clin Res Hepatol Gastroenterol. 2011; 35:805–812.5. Gane EJ, Agarwal K. Directly acting antivirals (DAAs) for the treatment of chronic hepatitis C virus infection in liver transplant patients: "a flood of opportunity". Am J Transplant. 2014; 14:994–1002.6. Hsu SH, Yeh ML, Wang SN. New insights in recurrent HCV infection after liver transplantation. Clin Dev Immunol. 2013; 2013:890517.7. Roche B, Samuel D. Hepatitis C virus treatment pre- and post-liver transplantation. Liver Int. 2012; 32:120–128.8. Dhanasekaran R, Firpi RJ. Challenges of recurrent hepatitis C in the liver transplant patient. World J Gastroenterol. 2014; 20:3391–3400.9. Chalasani N, Manzarbeitia C, Ferenci P, Vogel W, Fontana RJ, Voigt M, Riely C, Martin P, Teperman L, Jiao J, et al. Pegasys Transplant Study Group. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology. 2005; 41:289–298.10. Bzowej N, Nelson DR, Terrault NA, Everson GT, Teng LL, Prabhakar A, Charlton MR. PHOENIX Study Group. PHOENIX: A randomized controlled trial of peginterferon alfa-2a plus ribavirin as a prophylactic treatment after liver transplantation for hepatitis C virus. Liver Transpl. 2011; 17:528–538.11. Shergill AK, Khalili M, Straley S, Bollinger K, Roberts JP, Ascher NA, Terrault NA. Applicability, tolerability and efficacy of preemptive antiviral therapy in hepatitis C-infected patients undergoing liver transplantation. Am J Transplant. 2005; 5:118–124.12. Sugawara Y, Makuuchi M, Matsui Y, Kishi Y, Akamatsu N, Kaneko J, Kokudo N. Preemptive therapy for hepatitis C virus after living-donor liver transplantation. Transplantation. 2004; 78:1308–1311.13. European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014; 60:392–420.14. Coilly A, Roche B, Duclos-Vallée JC, Samuel D. Management of HCV transplant patients with triple therapy. Liver Int. 2014; 34:46–52.15. Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007; 47:598–607.16. Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009; 49:1335–1374.17. Xirouchakis E, Triantos C, Manousou P, Sigalas A, Calvaruso V, Corbani A, Leandro G, Patch D, Burroughs A. Pegylated-interferon and ribavirin in liver transplant candidates and recipients with HCV cirrhosis: systematic review and meta-analysis of prospective controlled studies. J Viral Hepat. 2008; 15:699–709.18. Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Kremers WK, Rosen CB, Heimbach JK, Charlton MR. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant. 2008; 8:2426–2433.19. Berenguer M, Roche B, Aguilera V, Duclos-Vallée JC, Navarro L, Rubin A, Pons JA, de la Mata M, Prieto M, Samuel D. Efficacy of the retreatment of hepatitis C virus infections after liver transplantation: role of an aggressive approach. Liver Transpl. 2013; 19:69–77.20. Wang CS, Ko HH, Yoshida EM, Marra CA, Richardson K. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant. 2006; 6:1586–1599.21. Coto-Llerena M, Pérez-Del-Pulgar S, Crespo G, Carrión JA, Martinez SM, Sánchez-Tapias JM, Martorell J, Navasa M, Forns X. Donor and recipient IL28B polymorphisms in HCV-infected patients undergoing antiviral therapy before and after liver transplantation. Am J Transplant. 2011; 11:1051–1057.22. Peveling-Oberhag J, Zeuzem S, Hofmann WP. Antiviral therapy of chronic hepatitis C in patients with advanced liver disease and after liver transplantation. Med Microbiol Immunol. 2010; 199:1–10.23. Ciria R, Pleguezuelo M, Khorsandi SE, Davila D, Suddle A, Vilca-Melendez H, Rufian S, de la Mata M, Briceño J, Cillero PL, et al. Strategies to reduce hepatitis C virus recurrence after liver transplantation. World J Hepatol. 2013; 5:237–250.24. Jiménez-Pérez M, Garcia DM, Grande RG, Daga JA, Pulido LB, Aguilar MD, Bravo MA, López JM, de la Mata Garcia MM. Analysis of the recurrence of hepatitis C virus after liver transplantation: results of the Andalusian liver registry. Transplant Proc. 2013; 45:276–278.25. Gelley F, Gámán G, Gerlei Z, Zádori G, Görög D, Kóbori L, Fehervari I, Schuller J, Szőnyi L, Nagy P, et al. Hepatitis C virus recurrence after liver transplantation in Hungary. Trends over the past 10 years. Orv Hetil. 2013; 154:1058–1066.26. Schiano TD, Charlton M, Younossi Z, Galun E, Pruett T, Tur-Kaspa R, Eren R, Dagan S, Graham N, Williams PV, et al. Monoclonal antibody HCV-AbXTL68 in patients undergoing liver transplantation for HCV: results of a phase 2 randomized study. Liver Transpl. 2006; 12:1381–1389.27. Gurusamy KS, Tsochatzis E, Davidson BR, Burroughs AK. Antiviral prophylactic intervention for chronic hepatitis C virus in patients undergoing liver transplantation. Cochrane Database Syst Rev. 2010; CD006573.28. Davis GL, Nelson DR, Terrault N, Pruett TL, Schiano TD, Fletcher CV, Sapan CV, Riser LN, Li Y, Whitley RJ, et al. Collaborative Antiviral Study Group. A randomized, open-label study to evaluate the safety and pharmacokinetics of human hepatitis C immune globulin (Civacir) in liver transplant recipients. Liver Transpl. 2005; 11:941–949.29. Kim YS, Ahn YO, Lee HS. Risk factors for hepatitis C virus infection among Koreans according to the hepatitis C virus genotype. J Korean Med Sci. 2002; 17:187–192.30. Seong MH, Kil H, Kim YS, Bae SH, Lee YJ, Lee HC, Kang BH, Jeong SH. Clinical and epidemiological features of hepatitis C virus infection in South Korea: a prospective, multicenter cohort study. J Med Virol. 2013; 85:1724–1733.31. Kil H, Jeong SH, Kim JW, Byoun YS, Min BY, Woo BH, Lee YJ, Kim YS. Role of interleukin-28B genetic polymorphisms in Korean patients with hepatitis C virus infection. Gut Liver. 2014; 8:70–78.32. Lyoo K, Song MJ, Hur W, Choi JE, Hong SW, Kim CW, Bae SH, Choi JY, Choi SW, Shin EC, et al. Polymorphism near the IL28B gene in Korean hepatitis C virus-infected patients treated with peg-interferon plus ribavirin. J Clin Virol. 2011; 52:363–366.33. Sinn DH, Kim YJ, Lee ST, Gwak GY, Choi MS, Lee JH, Koh KC, Yoo BC, Paik SW. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in Asian patients. J Gastroenterol Hepatol. 2011; 26:1374–1379.34. Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009; 461:798–801.35. Firpi RJ, Zhu H, Morelli G, Abdelmalek MF, Soldevila-Pico C, Machicao VI, Cabrera R, Reed AI, Liu C, Nelson DR. Cyclosporine suppresses hepatitis C virus in vitro and increases the chance of a sustained virological response after liver transplantation. Liver Transpl. 2006; 12:51–57.36. Charlton MR, Thompson A, Veldt BJ, Watt K, Tillmann H, Poterucha JJ, Heimbach JK, Goldstein D, McHutchison J. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology. 2011; 53:317–324.37. Rabie R, Mumtaz K, Renner EL. Efficacy of antiviral therapy for hepatitis C after liver transplantation with cyclosporine and tacrolimus: a systematic review and meta-analysis. Liver Transpl. 2013; 19:36–48.38. Maticic M, Luznik Z, Stepec S, Popovic P, Snedec N, Poljak M, Stanisavljevic D. A modified ribavirin-free interferon therapy with boceprevir in post-liver transplant recurrent hepatitis C. J Clin Gastroenterol. 2014; 48:464–465.39. Fontana RJ, Hughes EA, Bifano M, Appelman H, Dimitrova D, Hindes R, Symonds WT. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am J Transplant. 2013; 13:1601–1605.40. Burton JR Jr, O'Leary JG, Verna EC, Saxena V, Dodge JL, Stravitz RT, Levitsky J, Trotter JF, Everson GT, Brown RS Jr, et al. A US multicenter study of hepatitis C treatment of liver transplant recipients with protease-inhibitor triple therapy. J Hepatol. 2014; 61:508–514.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Hepatitis C Viral Infection Pre- and Post-liver Transplantation
- Mechanims of Immune Responses and Liver Cell Injury in Response to Hepatitis B Virus Infection
- Prophylaxis Against Hepatitis B Recurrence Following Liver Transplantation
- Management of viral hepatitis in liver transplant recipients
- Prophylaxis against Hepatitis B Recurrence Following Liver Transplantation in HBsAg( ) Patients: Hepatitis B Immune Globulin vs Lamivudine