J Korean Med Sci.
2015 Nov;30(11):1553-1557. 10.3346/jkms.2015.30.11.1553.
Development of Quality Management Systems for Clinical Practice Guidelines in Korea
- Affiliations
-
- 1Department of Health Management and Policy, Kangwon National University, School of Medicine, Chuncheon, Korea.
- 2The Executive Committee for Clinical Practice Guideline, The Korean Academy of Medical Sciences, Seoul, Korea.
- 3Department of Radiology, Yonsei University Sevrance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Urology, Kyung Hee University Medical Center, School of Medicine Kyung-Hee University, Seoul, Korea.
- 5Research Agency for Clinical Practice Guidelines, KAMS Research Center, The Korean Academy of Medical Sciences, Seoul, Korea.
- 6Department of Preventive Medicine, Kangwon National University Hospital, Chuncheon, Korea. somanghana@gmail.com
- KMID: 2351103
- DOI: http://doi.org/10.3346/jkms.2015.30.11.1553
Abstract
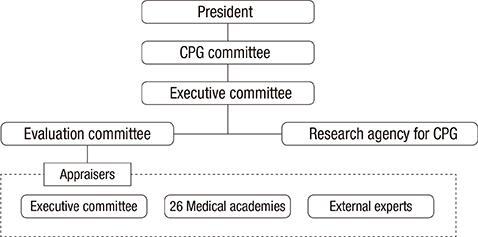
- This study introduces the Clinical practice guidelines (CPGs) appraisal system by the Korean Academy of Medical Sciences (KAMS). Quality management policies for CPGs vary among different countries, which have their own cultures and health care systems. However, supporting developers in guideline development and appraisals using standardized tools are common practices. KAMS, an organization representing the various medical societies of Korea, has been striving to establish a quality management system for CPGs, and has established a CPGs quality management system that reflects the characteristics of the Korean healthcare environment and the needs of its users. KAMS created a foundation for the development of CPGs, set up an independent appraisal organization, enacted regulations related to the appraisals, and trained appraisers. These efforts could enhance the ability of each individual medical society to develop CPGs, to increase the quality of the CPGs, and to ultimately improve the quality of the information available to decision-makers.
Keyword
MeSH Terms
Figure
Reference
-
1. Darling G. The impact of clinical practice guidelines and clinical trials on treatment decisions. Surg Oncol. 2002; 11:255–262.2. Legido-Quigley H, Panteli D, Brusamento S, Knai C, Saliba V, Turk E, Solé M, Augustin U, Car J, McKee M, et al. Clinical guidelines in the European Union: mapping the regulatory basis, development, quality control, implementation and evaluation across member states. Health Policy. 2012; 107:146–156.3. Jo HS. Current status of development of clinical practice guidelies in Korea and application for evidence based nursing. Korean Soc Evid Based Nurs. 2014; 2:1–4.4. Lee YK, Shin ES, Shim JY, Min KJ, Kim JM, Lee SH. Executive Committee for CPGs. Korean Academy of Medical Sciences. Developing a scoring guide for the Appraisal of Guidelines for Research and Evaluation II instrument in Korea: a modified Delphi consensus process. J Korean Med Sci. 2013; 28:190–194.5. Jo MW, Lee JY, Kim NS, Kim SY, Sheen S, Kim SH, Lee SI. Assessment of the quality of clinical practice guidelines in Korea using the AGREE Instrument. J Korean Med Sci. 2013; 28:357–365.6. Ahn HS, Kim HJ. Development and implementation of clinical practice guidelines: current status in Korea. J Korean Med Sci. 2012; 27:S55–S60.7. OECD Health Division. OECD Heath Care Quality Review. Korea, Assessment and Recommendation. Paris: OECD;2012. p. 13–14.8. AGREE in Europe Collaborative Group. Guideline development in Europe. An international comparison. Int J Technol Assess Health Care. 2000; 16:1039–1049.9. Kelley E, Moy E, Kosiak B, McNeill D, Zhan C, Stryer D, Clancy C. Prevention health care quality in America: findings from the first National Healthcare Quality and Disparities reports. Prev Chronic Dis. 2004; 1:A03.10. Kim NS, Kim SY, Park EJ. Promoting the quality of medicine: based on clinical practice guidelines. Seoul, Korea: Korea Institue for Health and Social Affairs;2004.11. Graham ID, Beardall S, Carter AO, Glennie J, Hébert PC, Tetroe JM, McAlister FA, Visentin S, Anderson GM. What is the quality of drug therapy clinical practice guidelines in Canada? CMAJ. 2001; 165:157–163.12. Nothacker M, Muche-Borowski C, Kopp IB. Reflections on 20 years of clinical practice guideline programmes in Germany: what is their impact? Z Evid Fortbild Qual Gesundhwes. 2014; 108:550–559.13. Ollenschläger G, Kopp I, Lelgemann M, Sänger S, Heymans L, Thole H, Trapp H, Lorenz W, Selbmann HK, Encke A. The German program for disease management guidelines. Background, methods, and development process. Med Klin (Munich). 2006; 101:840–845.14. Canadian Medical Association. Criteria for inclusion of a CPG in the CMA Infobase. accessed on 12 May 2015. Available at https://www.cma.ca/En/Pages/submit-guideline.aspx.15. National Guideline Clearinghouse. National Guideline Clearinghouse Inclusion Criteria 2014. accessed on 12 May 2015. Available at http://www.guideline.gov/about/inclusion-criteria.aspx.16. Canadian Medical Association. Handbook on clinical practice guidelines. Ottawa, Canada: Canadian Medical Association;2007.17. National Institute for Health and Care Excellence. NICE Guidance 2015. accessed on 12 May 2015. Available at https://www.nice.org.uk/guidance/published.18. National Health Research and Medical Council. Procedures and requirements for meeting the 2011 NHMRC standard for clinical practice guidelines. Canberra, Australia: National Health and Medical Research;2011.19. Pelly JE, Newby L, Tito F, Redman S, Adrian AM. Clinical practice guidelines before the law: sword or shield? Med J Aust. 1998; 169:330–333.20. Korea Association of Medical Science. Korea Medical Guideline Information center. Seoul, Korea: accessed on 12 May 2015. Available at http://www.guideline.or.kr/contents/index.php?code=044.21. Kim YK, Lee SH, Seo JH, Kim JH, Kim SD, Kim GK. A comprehensive model of factors affecting adoption of clinical practice guidelines in Korea. J Korean Med Sci. 2010; 25:1568–1573.22. Yang J, Han C, Yoon HK, Pae CU, Kim MJ, Park SY, Ahn J. Experiences and barriers to implementation of clinical practice guideline for depression in Korea. BMC Psychiatry. 2013; 13:150.23. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010; 182:E839–E842.24. Oh MK, Jo H, Lee YK. Improving the reliability of clinical practice guideline appraisals: effects of the Korean AGREE II scoring guide. J Korean Med Sci. 2014; 29:771–775.25. Bussières A, Stuber K. The Clinical Practice Guideline Initiative: A joint collaboration designed to improve the quality of care delivered by doctors of chiropractic. J Can Chiropr Assoc. 2013; 57:279–284.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and Implementation of Clinical Practice Guidelines: Current Status in Korea
- Trend analysis of grading systems for level of evidence and strength of recommendation
- The Current Status of Development of Korean Clinical Practice Guidelines in Urology
- Clinical Practice Guidelines for the Management of Bacterial Meningitis in Adults in Korea
- Development and Evaluation of Nursing Practice Guidelines for Water Treatment System in Hemodialysis