Korean J Physiol Pharmacol.
2016 Sep;20(5):515-523. 10.4196/kjpp.2016.20.5.515.
Molecular association of CD98, CD29, and CD147 critically mediates monocytic U937 cell adhesion
- Affiliations
-
- 1Depatment of Genetic Engineering, Sungkyunkwan University, Suwon 16419, Korea. jaecho@skku.edu
- 2School of Systems Biomedical Science, Soongsil University, Seoul 06978, Korea.
- KMID: 2350508
- DOI: http://doi.org/10.4196/kjpp.2016.20.5.515
Abstract
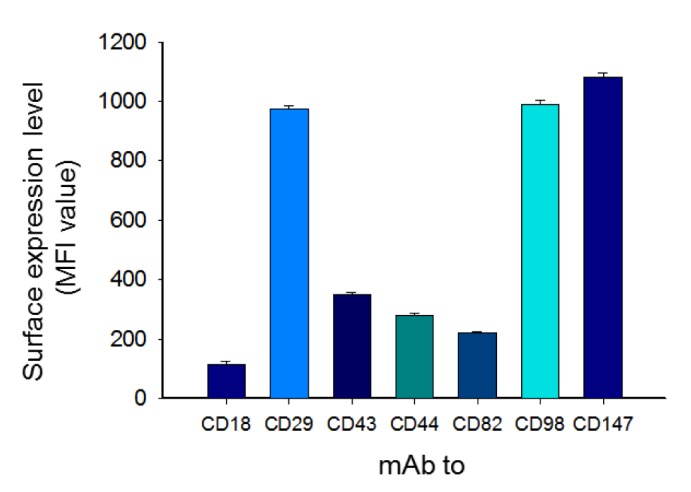
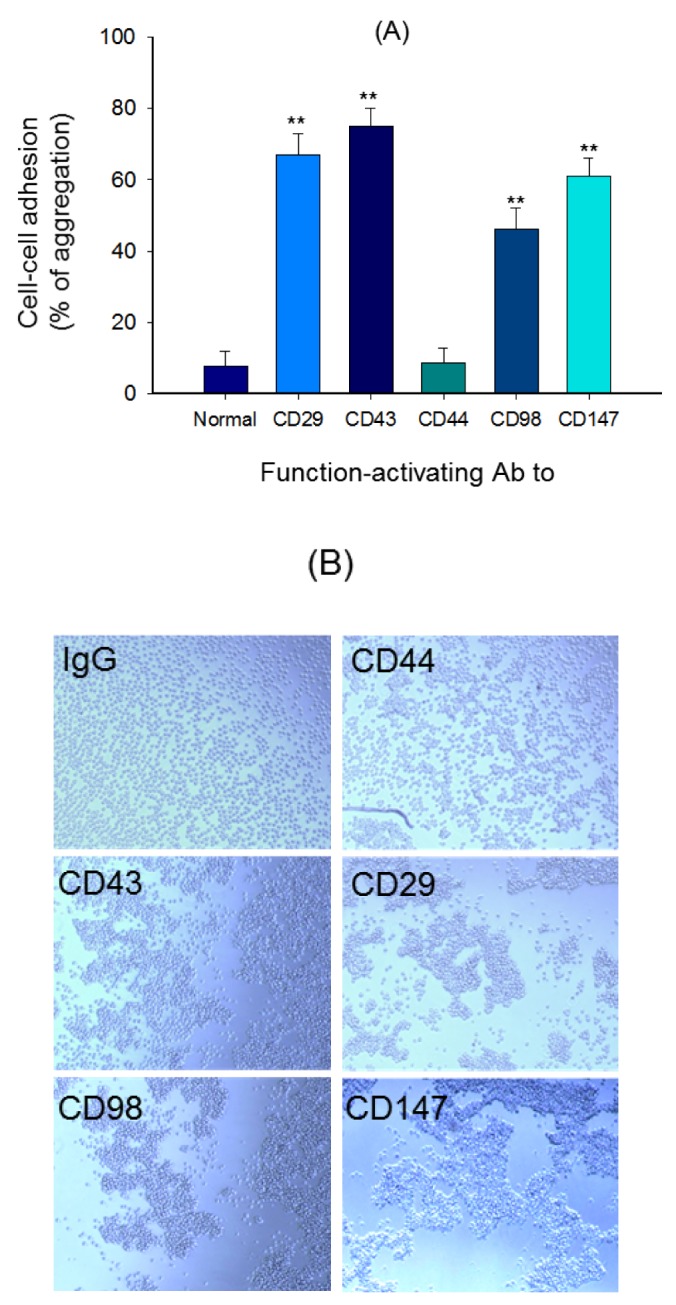
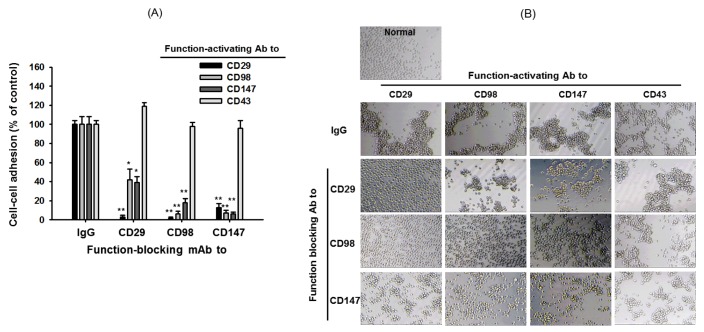
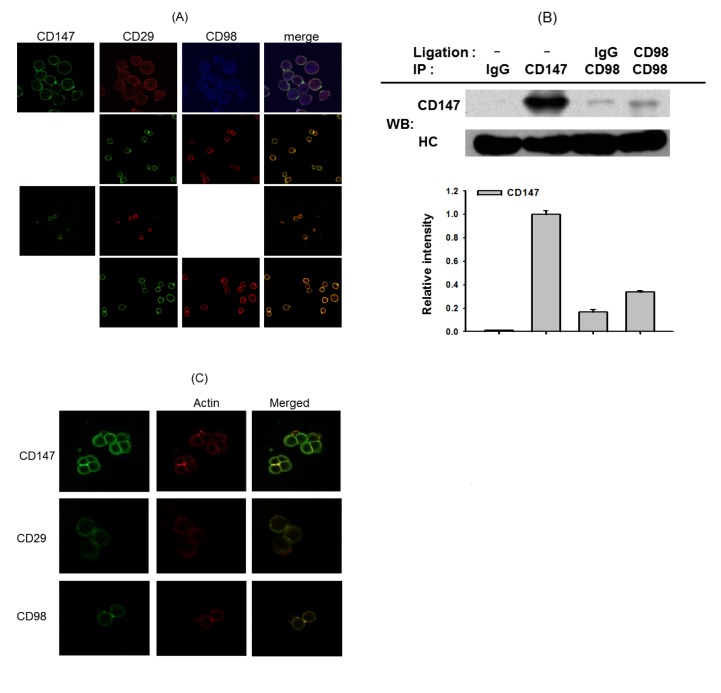
- Adhesion events of monocytes represent an important step in inflammatory responses induced by chemokines. The β1-integrin CD29 is a major adhesion molecule regulating leukocyte migration and extravasation. Although several adhesion molecules have been known as regulators of CD29, the molecular interactions between CD29 and its regulatory adhesion molecules (such as CD98 and CD147) have not been fully elucidated. Therefore, in this study, we examined whether these molecules are functionally, biochemically, and cell-biologically associated using monocytic U937 cells treated with aggregation-stimulating and blocking antibodies, as well as enzyme inhibitors. The surface levels of CD29, CD98, and CD147 (but not CD43, CD44, and CD82) were increased. The activation of CD29, CD98, and CD147 by ligation of them with aggregation-activating antibodies triggered the induction of cell-cell adhesion, and sensitivity to various enzyme inhibitors and aggregation-blocking antibodies was similar for CD29-, CD98-, and CD147-induced U937 cell aggregation. Molecular association between these molecules and the actin cytoskeleton was confirmed by confocal microscopy and immunoprecipitation. These results strongly suggest that CD29 might be modulated by its biochemical and cellular regulators, including CD98 and CD147, via the actin cytoskeleton.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014; 14:277–288. PMID: 25550694.
Article2. Yu T, Li YJ, Bian AH, Zuo HB, Zhu TW, Ji SX, Kong F, Yin de Q, Wang CB, Wang ZF, Wang HQ, Yang Y, Yoo BC, Cho JY. The regulatory role of activating transcription factor 2 in inflammation. Mediators Inflamm. 2014; 2014:950472. PMID: 25049453.
Article3. Ku SK, Bae JS. Baicalin, baicalein and wogonin inhibits high glucose-induced vascular inflammation in vitro and in vivo. BMB Rep. 2015; 48:519–524. PMID: 25739393.
Article4. Schnoor M, Alcaide P, Voisin MB, van Buul JD. Crossing the vascular wall: common and unique mechanisms exploited by different leukocyte subsets during extravasation. Mediators Inflamm. 2015; 2015:946509. PMID: 26568666.
Article5. Haertel B, von Woedtke T, Weltmann KD, Lindequist U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol Ther (Seoul). 2014; 22:477–490. PMID: 25489414.
Article6. Italiani P, Boraschi D. New insights into tissue macrophages: from their origin to the development of memory. Immune Netw. 2015; 15:167–176. PMID: 26330802.
Article7. Kim MJ, Rah SY, An JH, Kurokawa K, Kim UH, Lee BL. Human anti-peptidoglycan-IgG-mediated opsonophagocytosis is controlled by calcium mobilization in phorbol myristate acetate-treated U937 cells. BMB Rep. 2015; 48:36–41. PMID: 24856825.
Article8. Rintoul RC, Buttery RC, Mackinnon AC, Wong WS, Mosher D, Haslett C, Sethi T. Cross-linking CD98 promotes integrin-like signaling and anchorage-independent growth. Mol Biol Cell. 2002; 13:2841–2852. PMID: 12181350.
Article9. Hirai F, Nakayamada S, Okada Y, Saito K, Kurose H, Mogami A, Tanaka Y. Small GTPase Rho signaling is involved in beta1 integrin-mediated up-regulation of intercellular adhesion molecule 1 and receptor activator of nuclear factor kappaB ligand on osteoblasts and osteoclast maturation. Biochem Biophys Res Commun. 2007; 356:279–285. PMID: 17349971.10. McNally AK, Anderson JM. Beta1 and beta2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am J Pathol. 2002; 160:621–630. PMID: 11839583.11. Fulcher JA, Chang MH, Wang S, Almazan T, Hashimi ST, Eriksson AU, Wen X, Pang M, Baum LG, Singh RR, Lee B. Galectin-1 co-clusters CD43/CD45 on dendritic cells and induces cell activation and migration through Syk and protein kinase C signaling. J Biol Chem. 2009; 284:26860–26870. PMID: 19635795.
Article12. del Rio R, Rincón M, Layseca-Espinosa E, Fierro NA, Rosenstein Y, Pedraza-Alva G. PKCtheta is required for the activation of human T lymphocytes induced by CD43 engagement. Biochem Biophys Res Commun. 2004; 325:133–143. PMID: 15522211.13. Cho JY, Katz DR, Skubitz KM, Chain BM. Conventional protein kinase C plays a critical role in negative regulation of CD98-induced homotypic aggregation. Tissue Antigens. 2010; 75:19–29. PMID: 19895572.
Article14. Cho JY, Skubitz KM, Katz DR, Chain BM. CD98-dependent homotypic aggregation is associated with translocation of protein kinase Cdelta and activation of mitogen-activated protein kinases. Exp Cell Res. 2003; 286:1–11. PMID: 12729789.15. Khayati F, Pérez-Cano L, Maouche K, Sadoux A, Boutalbi Z, Podgorniak MP, Maskos U, Setterblad N, Janin A, Calvo F, Lebbé C, Menashi S, Fernandez-Recio J, Mourah S. EMMPRIN/CD147 is a novel coreceptor of VEGFR-2 mediating its activation by VEGF. Oncotarget. 2015; 6:9766–9780. PMID: 25825981.
Article16. Xu T, Zhou M, Peng L, Kong S, Miao R, Shi Y, Sheng H, Li L. Upregulation of CD147 promotes cell invasion, epithelial-to-mesenchymal transition and activates MAPK/ERK signaling pathway in colorectal cancer. Int J Clin Exp Pathol. 2014; 7:7432–7441. PMID: 25550778.17. Wang Y, Yuan L, Yang XM, Wei D, Wang B, Sun XX, Feng F, Nan G, Wang Y, Chen ZN, Bian H. A chimeric antibody targeting CD147 inhibits hepatocellular carcinoma cell motility via FAK-PI3K-Akt-Girdin signaling pathway. Clin Exp Metastasis. 2015; 32:39–53. PMID: 25424030.
Article18. Langereis JD. Neutrophil integrin affinity regulation in adhesion, migration, and bacterial clearance. Cell Adh Migr. 2013; 7:476–481. PMID: 24430200.
Article19. Sun C, Zargham R, Shao Q, Gui X, Marcus V, Lazaris A, Salman A, Metrakos P, Qu X, Gao Z. Association of CD98, integrin β1, integrin β3 and Fak with the progression and liver metastases of colorectal cancer. Pathol Res Pract. 2014; 210:668–674. PMID: 25041835.
Article20. Prager GW, Féral CC, Kim C, Han J, Ginsberg MH. CD98hc (SLC3A2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling. J Biol Chem. 2007; 282:24477–24484. PMID: 17597067.21. Cho JY, Fox DA, Horejsi V, Sagawa K, Skubitz KM, Katz DR, Chain B. The functional interactions between CD98, beta1-integrins, and CD147 in the induction of U937 homotypic aggregation. Blood. 2001; 98:374–382. PMID: 11435306.22. Guo N, Zhang K, Lv M, Miao J, Chen Z, Zhu P. CD147 and CD98 complex-mediated homotypic aggregation attenuates the CypA-induced chemotactic effect on Jurkat T cells. Mol Immunol. 2015; 63:253–263. PMID: 25089027.
Article23. Fei F, Li X, Xu L, Li D, Zhang Z, Guo X, Yang H, Chen Z, Xing J. CD147-CD98hc complex contributes to poor prognosis of non-small cell lung cancer patients through promoting cell proliferation via the PI3K/Akt signaling pathway. Ann Surg Oncol. 2014; 21:4359–4368. PMID: 25084765.
Article24. Kim MY, Yoo BC, Cho JY. Ginsenoside-Rp1-induced apolipoprotein A-1 expression in the LoVo human colon cancer cell line. J Ginseng Res. 2014; 38:251–255. PMID: 25379004.
Article25. Lee YG, Lee J, Cho JY. Cell-permeable ceramides act as novel regulators of U937 cell-cell adhesion mediated by CD29, CD98, and CD147. Immunobiology. 2010; 215:294–303. PMID: 19576658.
Article26. Kim S, Oh MH, Kim BS, Kim WI, Cho HS, Park BY, Park C, Shin GW, Kwon J. Upregulation of heme oxygenase-1 by ginsenoside Ro attenuates lipopolysaccharide-induced inflammation in macrophage cells. J Ginseng Res. 2015; 39:365–370. PMID: 26869829.
Article27. Wang Z, Wang D, Li Y, Zhang X. Protective effects of verapamil against H2O2-induced apoptosis in human lens epithelial cells. Biomol Ther (Seoul). 2014; 22:553–557. PMID: 25489424.
Article28. Baek KS, Hong YD, Kim Y, Sung NY, Yang S, Lee KM, Park JY, Park JS, Rho HS, Shin SS, Cho JY. Anti-inflammatory activity of AP-SF, a ginsenoside-enriched fraction, from Korean ginseng. J Ginseng Res. 2015; 39:155–161. PMID: 26045689.
Article29. Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015; 107:321–330. PMID: 25990461.
Article30. Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002; 22:146–167. PMID: 11857637.
Article31. Li Y, Wu J, Song F, Tang J, Wang SJ, Yu XL, Chen ZN, Jiang JL. Extracellular membrane-proximal domain of HAb18G/CD147 binds to metal ion-dependent adhesion site (MIDAS) motif of integrin β1 to modulate malignant properties of hepatoma cells. J Biol Chem. 2012; 287:4759–4772. PMID: 22130661.
Article32. Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997; 390:81–85. PMID: 9363894.
Article33. Mattila PK, Batista FD, Treanor B. Dynamics of the actin cytoskeleton mediates receptor cross talk: an emerging concept in tuning receptor signaling. J Cell Biol. 2016; 212:267–280. PMID: 26833785.
Article34. Kim JH, Lee YG, Yoo S, Oh J, Jeong D, Song WK, Yoo BC, Rhee MH, Park J, Cha SH, Hong S, Cho JY. Involvement of Src and the actin cytoskeleton in the antitumorigenic action of adenosine dialdehyde. Biochem Pharmacol. 2013; 85:1042–1056. PMID: 23353701.
Article35. Kim JY, Lee YG, Kim MY, Byeon SE, Rhee MH, Park J, Katz DR, Chain BM, Cho JY. Src-mediated regulation of inflammatory responses by actin polymerization. Biochem Pharmacol. 2010; 79:431–443. PMID: 19769947.
Article36. Cho JY, Kim AR, Joo HG, Kim BH, Rhee MH, Yoo ES, Katz DR, Chain BM, Jung JH. Cynaropicrin, a sesquiterpene lactone, as a new strong regulator of CD29 and CD98 functions. Biochem Biophys Res Commun. 2004; 313:954–961. PMID: 14706635.
Article37. Bruck R, Hershkoviz R, Lider O, Shirin H, Aeed H, Halpern Z. The use of synthetic analogues of Arg-Gly-Asp (RGD) and soluble receptor of tumor necrosis factor to prevent acute and chronic experimental liver injury. Yale J Biol Med. 1997; 70:391–402. PMID: 9626759.38. Park EJ, Yuki Y, Kiyono H, Shimaoka M. Structural basis of blocking integrin activation and deactivation for anti-inflammation. J Biomed Sci. 2015; 22:51. PMID: 26152212.
Article39. Nakayamada S, Saito K, Nakano K, Tanaka Y. Activation signal transduction by beta1 integrin in T cells from patients with systemic lupus erythematosus. Arthritis Rheum. 2007; 56:1559–1568. PMID: 17469136.40. Zhu Y, Feng Y, Liu H, Ye H, Guo C, Feng J, Dai S, Zheng X. CD4+CD29+T cells are blamed for the persistent inflammatory response in ulcerative colitis. Int J Clin Exp Pathol. 2015; 8:2627–2637. PMID: 26045768.41. Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler Thromb Vasc Biol. 2003; 23:1384–1390. PMID: 12805072.
Article42. Zickus C, Kunkel SL, Simpson K, Evanoff H, Glass M, Strieter RM, Lukacs NW. Differential regulation of C-C chemokines during fibroblast-monocyte interactions: adhesion vs. inflammatory cytokine pathways. Mediators Inflamm. 1998; 7:269–274. PMID: 9792337.
Article43. Miller LA, Hong JJ, Kinch MS, Harrison ML, Geahlen RL. The engagement of beta1 integrins on promonocytic cells promotes phosphorylation of Syk and formation of a protein complex containing Lyn and beta1 integrin. Eur J Immunol. 1999; 29:1426–1434. PMID: 10359096.44. da Costa Martins PA, van Gils JM, Mol A, Hordijk PL, Zwaginga JJ. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol. 2006; 79:499–507.45. Chiang YJ, Ho KC, Sun CT, Chiu JJ, Lee FJ, Liao F, Yang-Yen HF, Yen JJ. CBAP functions as a novel component in chemokine-induced ZAP70-mediated T-cell adhesion and migration. PLoS One. 2013; 8:e61761. PMID: 23620790.
Article46. Kim E, Yang WS, Kim JH, Park JG, Kim HG, Ko J, Hong YD, Rho HS, Shin SS, Sung GH, Cho JY. Lancemaside A from Codonopsis lanceolata modulates the inflammatory responses mediated by monocytes and macrophages. Mediators Inflamm. 2014; 2014:405158. PMID: 24782593.47. Jo S, Lee H, Kim S, Lee CH, Chung H. Korean red ginseng extract induces proliferation to differentiation transition of human acute promyelocytic leukemia cells via MYC-SKP2-CDKN1B axis. J Ethnopharmacol. 2013; 150:700–707. PMID: 24095829.
Article48. Herman SE, Mustafa RZ, Jones J, Wong DH, Farooqui M, Wiestner A. Treatment with Ibrutinib inhibits BTK- and VLA-4-dependent adhesion of chronic lymphocytic leukemia cells in vivo. Clin Cancer Res. 2015; 21:4642–4651. PMID: 26089373.
Article49. Wang H, Wu C, Wan S, Zhang H, Zhou S, Liu G. Shikonin attenuates lung cancer cell adhesion to extracellular matrix and metastasis by inhibiting integrin β1 expression and the ERK1/2 signaling pathway. Toxicology. 2013; 308:104–112. PMID: 23562787.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD98 activation increases surface expression and clusteringof beta 1 integrins in MCF-7 cells through FAK/Src- and cytoskeleton-independent mechanisms
- CD98 Activation Increases the Invasion of Human Breast Carcinoma MCF-7 Cells
- Effect of Eicosapentaenoic Acid on Endothelial Cell-U937 Cell Adhesion
- The Stimulation of CD147 Induces MMP-9 Expression through ERK and NF-kappaB in Macrophages: Implication for Atherosclerosis
- Identification and Functional Characterization of Differentially Expressed Genes in Human-derived Monocytic Cell Line U937 Infected with Mycobacterium tuberculosis H37Rv and Mycobacterium marinum: Comparative Evaluation of IL-8