Korean J Physiol Pharmacol.
2016 Sep;20(5):449-457. 10.4196/kjpp.2016.20.5.449.
N-acetyl-L-cysteine and cysteine increase intracellular calcium concentration in human neutrophils
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Hallym University, Chuncheon 24252, Korea. dksong@hallym.ac.kr
- KMID: 2350501
- DOI: http://doi.org/10.4196/kjpp.2016.20.5.449
Abstract
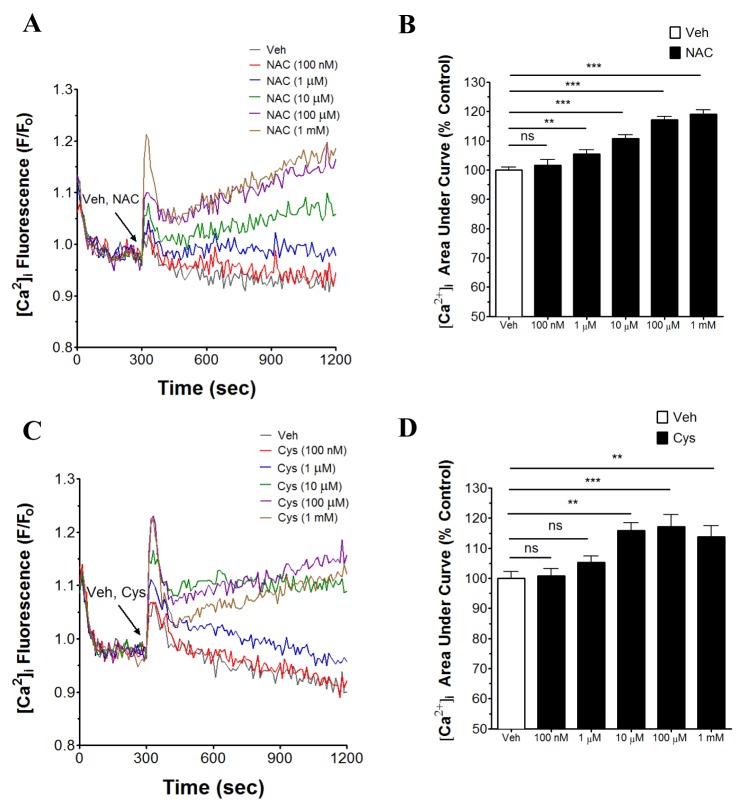
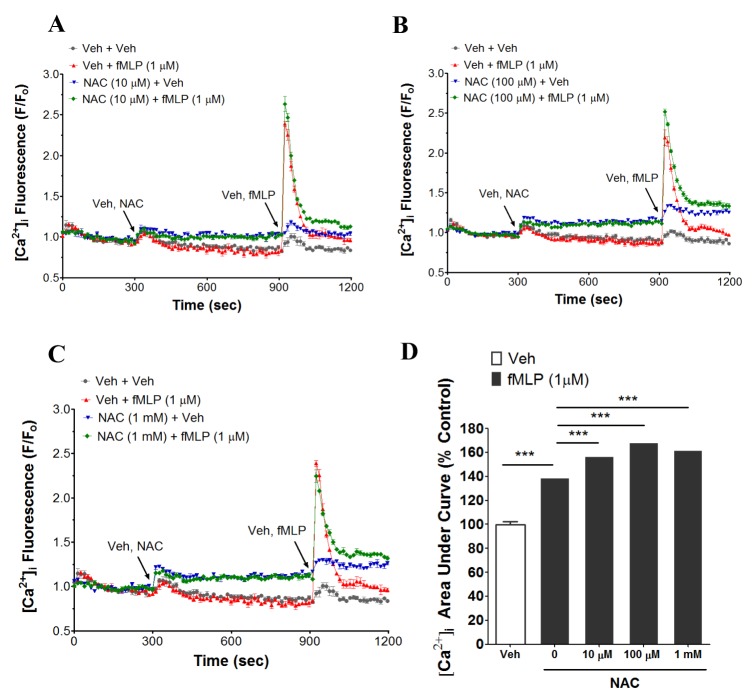
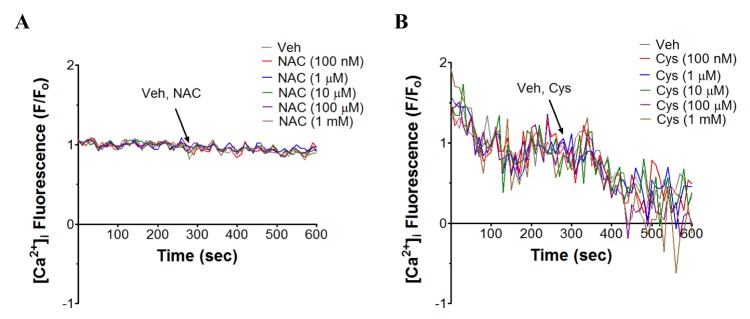
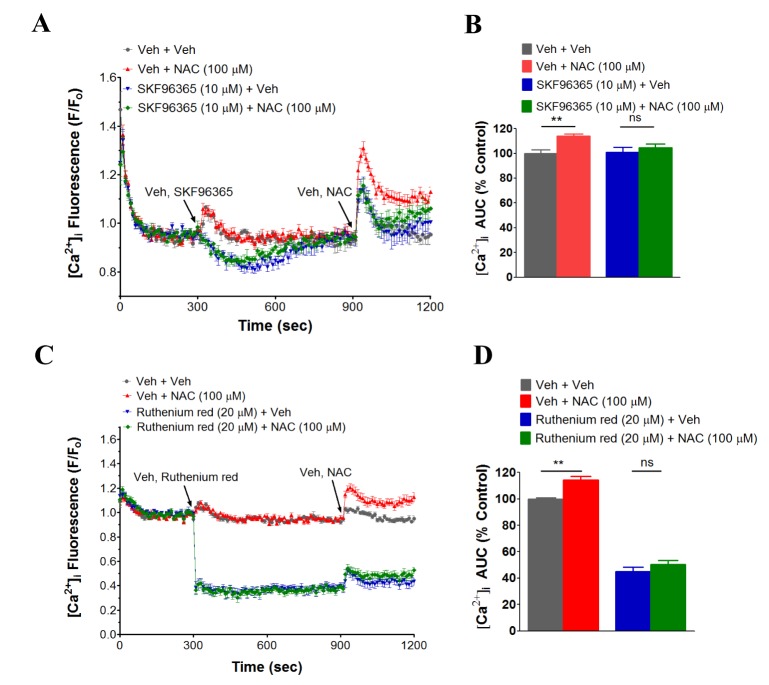
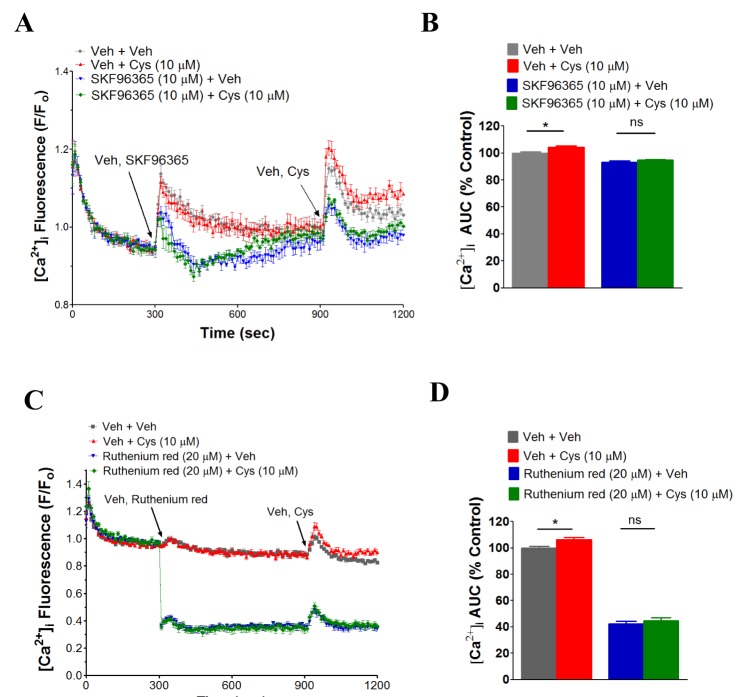
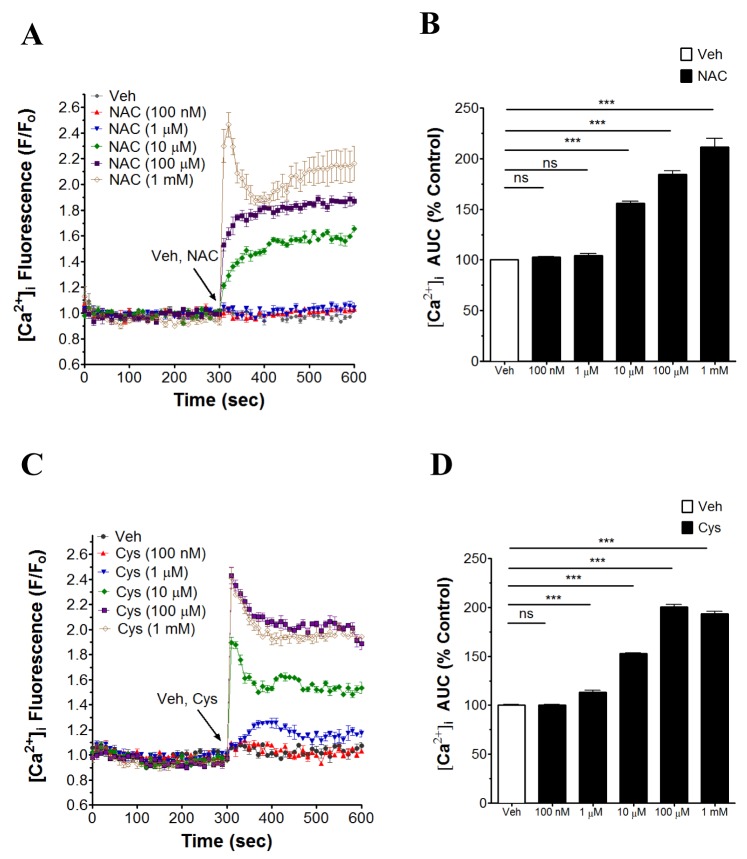
- N-acetyl-L-cysteine (NAC) and cysteine have been implicated in a number of human neutrophils' functional responses. However, though Ca²âº signaling is one of the key signalings contributing to the functional responses of human neutrophils, effects of NAC and cysteine on intracellular calcium concentration ([Ca²âº]áµ¢) in human neutrophils have not been investigated yet. Thus, this study was carried out with an objective to investigate the effects of NAC and cysteine on [Ca²âº]áµ¢ in human neutrophils. We observed that NAC (1 µM ~ 1 mM) and cysteine (10 µM ~ 1 mM) increased [Ca²âº]áµ¢ in human neutrophils in a concentration-dependent manner. In NAC pre-supplmented buffer, an additive effect on N-formyl-methionine-leucine-phenylalanine (fMLP)-induced increase in [Ca²âº]áµ¢ in human neutrophils was observed. In Ca²âº-free buffer, NAC- and cysteine-induced [Ca²âº]áµ¢ increase in human neutrophils completely disappeared, suggesting that NAC- and cysteine-mediated increase in [Ca²âº]áµ¢ in human neutrophils occur through Ca²âº influx. NAC- and cysteine-induced [Ca²âº]áµ¢ increase was effectively inhibited by calcium channel inhibitors SKF96365 (10 µM) and ruthenium red (20 µM). In Naâº-free HEPES, both NAC and cysteine induced a marked increase in [Ca²âº]áµ¢ in human neutrophils, arguing against the possibility that Naâº-dependent intracellular uptake of NAC and cysteine is necessary for their [Ca²âº]áµ¢ increasing activity. Our results show that NAC and cysteine induce [Ca²âº]áµ¢ increase through Ca²âº influx in human neutrophils via SKF96365- and ruthenium red-dependent way.
MeSH Terms
Figure
Reference
-
1. Murray RK, Granner DK, Mayes PA, Rodweil VW. Biosynthesis of nutritionally nonessential amino acids. Harper's Biochemistry. 22nd ed. USA: Appleton & Lange;1991. p. 267–269.2. Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998; 3:114–127. PMID: 9577247.3. De Flora S, Balansky RM, Bennicelli C, Camoirano A, D'agostini F, Izzotti A, Cesarone CF. Mechanisms of anticarcinogenesis: The example of N-acetylcysteine. In : Ioannides C, Lewis DFV, editors. Drugs Diet and Disease. Mechanistic Approaches to Cancer. Hemel Hempstead, UK: Ellis Horwood;1995. p. 151–203.4. Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999; 27:916–921. PMID: 10569624.
Article5. Aitio ML. N-acetylcysteine -- passe-partout or much ado about nothing? Br J Clin Pharmacol. 2006; 61:5–15. PMID: 16390346.
Article6. De Flora S, Izzotti A, D'agostini F, Balansky RM. Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking-related end-points. Carcinogenesis. 2001; 22:999–1013. PMID: 11408342.
Article7. Kobayashi SD, Voyich JM, Burlak C, Deleo FR. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz). 2005; 53:505–517. PMID: 16407783.8. Paulsen O, Forsgren A. Effects of N-acetylcysteine on human polymorphonuclear leukocytes. APMIS. 1989; 97:115–119. PMID: 2537647.
Article9. Jensen T, Kharazmi A, Schiøtz PO, Nielsen H, Stenvang Pedersen S, Stafanger G, Koch C, Høiby N. Effect of oral N-acetylcysteine administration on human blood neutrophil and monocyte function. APMIS. 1988; 96:62–67. PMID: 3345250.
Article10. Bernard GR, Lucht WD, Niedermeyer ME, Snapper JR, Ogletree ML, Brigham KL. Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J Clin Invest. 1984; 73:1772–1784. PMID: 6725559.
Article11. Kharazmi A, Nielsen H, Schiøtz PO. N-acetylcysteine inhibits human neutrophil and monocyte chemotaxis and oxidative metabolism. Int J Immunopharmacol. 1988; 10:39–46. PMID: 3366508.
Article12. Ohman L, Dahlgren C, Follin P, Lew D, Stendahl O. N-acetylcysteine enhances receptor-mediated phagocytosis by human neutrophils. Agents Actions. 1992; 36:271–277.13. Roberts RL, Aroda VR, Ank BJ. N-acetylcysteine enhances antibody-dependent cellular cytotoxicity in neutrophils and mononuclear cells from healthy adults and human immunodeficiency virus-infected patients. J Infect Dis. 1995; 172:1492–1502. PMID: 7594708.
Article14. Krump E, Pouliot M, Naccache PH, Borgeat P. Leukotriene synthesis in calcium-depleted human neutrophils: arachidonic acid release correlates with calcium influx. Biochem J. 1995; 310:681–688. PMID: 7654211.
Article15. Nüsse O, Serrander L, Foyouzi-Youssefi R, Monod A, Lew DP, Krause KH. Store-operated Ca2+ influx and stimulation of exocytosis in HL-60 granulocytes. J Biol Chem. 1997; 272:28360–28367. PMID: 9353293.16. Nüsse O, Serrander L, Lew DP, Krause KH. Ca2+-induced exocytosis in individual human neutrophils: high- and low-affinity granule populations and submaximal responses. EMBO J. 1998; 17:1279–1288. PMID: 9482725.17. Thelen M, Dewald B, Baggiolini M. Neutrophil signal transduction and activation of the respiratory burst. Physiol Rev. 1993; 73:797–821. PMID: 8415926.
Article18. Granfeldt D, Samuelsson M, Karlsson A. Capacitative Ca2+ influx and activation of the neutrophil respiratory burst. Different regulation of plasma membrane- and granule-localized NADPH-oxidase. J Leukoc Biol. 2002; 71:611–617. PMID: 11927647.19. van Kooyk Y, Weder P, Heije K, de Waal Malefijt R, Figdor CG. Role of intracellular Ca2+ levels in the regulation of CD11a/CD18 mediated cell adhesion. Cell Adhes Commun. 1993; 1:21–32. PMID: 7915956.20. Lawson MA, Maxfield FR. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995; 377:75–79. PMID: 7544874.21. Tintinger G, Steel HC, Anderson R. Taming the neutrophil: calcium clearance and influx mechanisms as novel targets for pharmacological control. Clin Exp Immunol. 2005; 141:191–200. PMID: 15996182.
Article22. Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013; 17:638–650. PMID: 23994464.
Article23. Merritt JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarthy SA, Moores KE, Rink TJ. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990; 271:515–522. PMID: 2173565.24. Zhu X, Jiang M, Birnbaumer L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)293 cells. Evidence for a non-capacitative Ca2+ entry. J Biol Chem. 1998; 273:133–142. PMID: 9417057.25. Shlykov SG, Yang M, Alcorn JL, Sanborn BM. Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod. 2003; 69:647–655. PMID: 12700192.27. Rychkov G, Barritt GJ. TRPC1 Ca2+-permeable channels in animal cells. Handb Exp Pharmacol. 2007; (179):23–52. PMID: 17217049.28. Bréchard S, Melchior C, Plançon S, Schenten V, Tschirhart EJ. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008; 44:492–506. PMID: 18436303.29. Singh A, Hildebrand ME, Garcia E, Snutch TP. The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br J Pharmacol. 2010; 160:1464–1475. PMID: 20590636.
Article30. Colton CK, Zhu MX. 2-Aminoethoxydiphenyl borate as a common activator of TRPV1, TRPV2, and TRPV3 channels. Handb Exp Pharmacol. 2007; (179):173–187. PMID: 17217057.
Article31. Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem J. 2001; 354:285–290. PMID: 11171105.32. Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol. 2001; 536:3–19. PMID: 11579153.33. Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002; 16:1145–1150. PMID: 12153982.34. Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005; 145:405–414. PMID: 15806115.
Article35. Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgårdh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006; 98:557–563. PMID: 16439693.
Article36. Bréchard S, Melchior C, Plançon S, Schenten V, Tschirhart EJ. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008; 44:492–506. PMID: 18436303.37. Sandmann S, Unger T. L- and T-type calcium channel blockade -the efficacy of the calcium channel antagonist mibefradil. J Clin Basic Cardiol. 1999; 2:187–201.38. Hill K, Benham CD, Mcnulty S, Randall AD. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology. 2004; 47:450–460. PMID: 15275834.
Article39. Hill K, Mcnulty S, Randall AD. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch Pharmacol. 2004; 370:227–237. PMID: 15549272.
Article40. Reid K, Guo TZ, Davies MF, Maze M. Nifedipine, an L-type calcium channel blocker, restores the hypnotic response in rats made tolerant to the alpha-2 adrenergic agonist dexmedetomidine. J Pharmacol Exp Ther. 1997; 283:993–999. PMID: 9399968.41. Seres T, Knickelbein RG, Warshaw JB, Johnston RB Jr. The phagocytosis-associated respiratory burst in human monocytes is associated with increased uptake of glutathione. J Immunol. 2000; 165:3333–3340. PMID: 10975851.
Article42. Young JD, Wolowyk MW, Jones SE, Ellory JC. Sodium-dependent cysteine transport in human red blood cells. Nature. 1979; 279:800–802. PMID: 450132.
Article43. Shanker G, Allen JW, Mutkus LA, Aschner M. The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res. 2001; 902:156–163. PMID: 11384608.
Article44. Elferink JG, de Koster BM. N-acetylcysteine causes a transient stimulation of neutrophil migration. Immunopharmacology. 1998; 38:229–236. PMID: 9506822.
Article45. Bei L, Hu T, Qian ZM, Shen X. Extracellular Ca2+ regulates the respiratory burst of human neutrophils. Biochim Biophys Acta. 1998; 1404:475–483. PMID: 9739175.46. Lindemann O, Strodthoff C, Horstmann M, Nielsen N, Jung F, Schimmelpfennig S, Heitzmann M, Schwab A. TRPC1 regulates fMLP-stimulated migration and chemotaxis of neutrophil granulocytes. Biochim Biophys Acta. 2015; 1853:2122–2130. PMID: 25595528.
Article47. Niessen HW, Kuijpers TW, Roos D, Verhoeven AJ. Release of azurophilic granule contents in fMLP-stimulated neutrophils requires two activation signals, one of which is a rise in cytosolic free Ca2+. Cell Signal. 1991; 3:625–633. PMID: 1786209.48. Sadowska AM, Manuel-y-keenoy B, Vertongen T, Schippers G, Radomska-Lesniewska D, Heytens E, De Backer WA. Effect of N-acetylcysteine on neutrophil activation markers in healthy volunteers: in vivo and in vitro study. Pharmacol Res. 2006; 53:216–225. PMID: 16384711.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of N-acetyl-L-cysteine and Glutathione on Antioxidant Status of Human Serum and 3T3 Fibroblasts
- Mechanism of an increase in concentration of intracellular calcium by carbachol in human gastric smooth muscle cell
- Significance of Secreted Protein Acidic and Rich in Cysteine Expression in Colorectal Carcinoma
- The antioxidant roles of L-carnitine and N-acetyl cysteine against oxidative stress on human sperm functional parameters during vitrification
- Cysteine as a potential donor urinary biomarker for donor acute kidney injury and recipient early graft function