Immune Netw.
2016 Aug;16(4):242-248. 10.4110/in.2016.16.4.242.
The Anti-apoptotic Effect of Ghrelin on Restraint Stress-Induced Thymus Atrophy in Mice
- Affiliations
-
- 1Global Research Laboratory, Department of Biochemistry and Molecular Biology, College of Medicine, Korea University, Seoul 02841, Korea. immunojun@korea.ac.kr, kyunglee@korea.ac.kr
- 2Department of Biomedical Science, College of Life Science, CHA University, Seongnam 13524, Korea.
- 3Department of Anatomy, School of Medicine, Ajou University, Suwon 16499, Korea.
- 4Department of Immunology, School of Medicine, Konkuk University, Chungju 27478, Korea.
- KMID: 2349885
- DOI: http://doi.org/10.4110/in.2016.16.4.242
Abstract
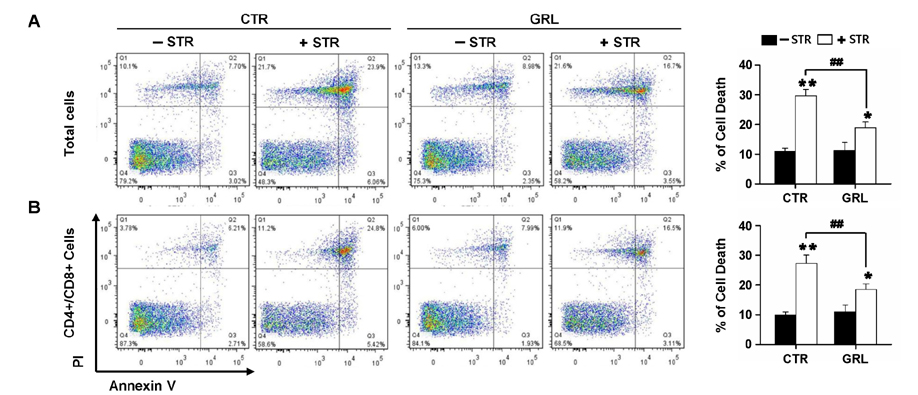
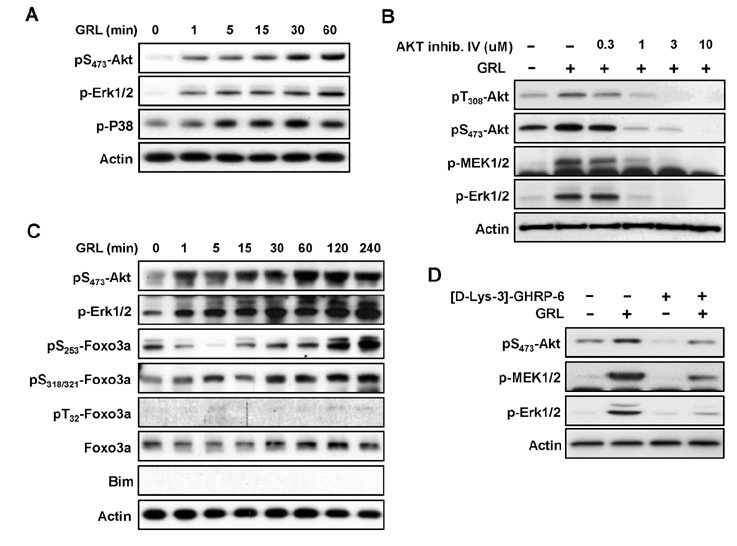
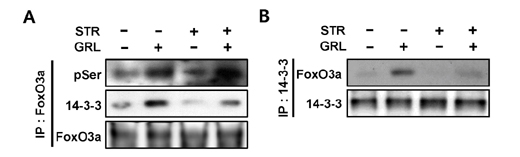
- Thymic atrophy is a complication that results from exposure to many environmental stressors, disease treatments, and microbial challenges. Such acute stress-associated thymic loss can have a dramatic impact on the host's ability to replenish the necessary naïve T cell output to reconstitute the peripheral T cell numbers and repertoire to respond to new antigenic challenges. We have previously reported that treatment with the orexigenic hormone ghrelin results in an increase in the number and proliferation of thymocytes after dexamethasone challenge, suggesting a role for ghrelin in restraint stress-induced thymic involution and cell apoptosis and its potential use as a thymostimulatory agent. In an effort to understand how ghrelin suppresses thymic T cell apoptosis, we have examined the various signaling pathways induced by receptor-specific ghrelin stimulation using a restraint stress mouse model. In this model, stress-induced apoptosis in thymocytes was effectively blocked by ghrelin. Western blot analysis demonstrated that ghrelin prevents the cleavage of pro-apoptotic proteins such as Bim, Caspase-3, and PARP. In addition, ghrelin stimulation activates the Akt and Mitogen-activated protein kinases (MAPK) signaling pathways in a time/dose-dependent manner. Moreover, we also revealed the involvement of the FoxO3a pathway in the phosphorylation of Akt and ERK1/2. Together, these findings suggest that ghrelin inhibits apoptosis by modulating the stress-induced apoptotic signal pathway in the restraint-induced thymic apoptosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986; 75:43–88.
Article2. Taub DD, Murphy WJ, Longo DL. Rejuvenation of the aging thymus: growth hormone-mediated and ghrelin-mediated signaling pathways. Curr Opin Pharmacol. 2010; 10:408–424.
Article3. Berczi I, Quintanar-Stephano A, Kovacs K. Neuroimmune regulation in immunocompetence, acute illness, and healing. Ann N Y Acad Sci. 2009; 1153:220–239.
Article4. Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008; 84:915–923.
Article5. Billard MJ, Gruver AL, Sempowski GD. Acute endotoxin-induced thymic atrophy is characterized by intrathymic inflammatory and wound healing responses. PLoS One. 2011; 6:e17940.
Article6. Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994; 152:5014–5021.7. Lee JH, Patel K, Tae HJ, Lustig A, Kim JW, Mattson MP, Taub DD. Ghrelin augments murine T-cell proliferation by activation of the phosphatidylinositol-3-kinase, extracellular signal-regulated kinase and protein kinase C signaling pathways. FEBS Lett. 2014; 588:4708–4719.
Article8. Baatar D, Patel K, Taub DD. The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol. 2011; 340:44–58.
Article9. Prodam F, Filigheddu N. Ghrelin gene products in acute and chronic inflammation. Arch Immunol Ther Exp (Warsz). 2014; 62:369–384.
Article10. DeBoer MD. The use of ghrelin and ghrelin receptor agonists as a treatment for animal models of disease: efficacy and mechanism. Curr Pharm Des. 2012; 18:4779–4799.
Article11. Lee JH, Halperin-Sheinfeld M, Baatar D, Mughal MR, Tae HJ, Kim JW, Carter A, Lustig A, Snir O, Lavie G, Okun E, Mattson MP, Sredni B, Taub DD. Tellurium compound AS101 ameliorates experimental autoimmune encephalomyelitis by VLA-4 inhibition and suppression of monocyte and T cell infiltration into the CNS. Neuromolecular Med. 2014; 16:292–307.
Article12. Tesauro M, Schinzari F, Caramanti M, Lauro R, Cardillo C. Cardiovascular and metabolic effects of ghrelin. Curr Diabetes Rev. 2010; 6:228–235.
Article13. Zhang GG, Cai HQ, Li YH, Sui YB, Zhang JS, Chang JR, Ning M, Wu Y, Tang CS, Qi YF, Yin XH. Ghrelin protects heart against ERS-induced injury and apoptosis by activating AMP-activated protein kinase. Peptides. 2013; 48:156–165.
Article14. Baldelli R, Bellone S, Broglio F, Ghigo E, Bona G. Ghrelin: a new hormone with endocrine and non-endocrine activities. Pediatr Endocrinol Rev. 2004; 2:8–14.15. Veldhuis JD, Bowers CY. Integrating GHS into the Ghrelin System. Int J Pept. 2010; pii: 879503.
Article16. Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007; 117:2778–2790.
Article17. El-Missiry MA. Antioxidant Enzyme. Rijeka, Croatia: InTech;2012. p. 303–320.18. Huang H, Tindall DJ. FOXO factors: a matter of life and death. Future Oncol. 2006; 2:83–89.
Article19. Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007; 120:2479–2487.
Article20. Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003; 73:689–701.
Article21. Patel K, Dixit VD, Lee JH, Kim JW, Schaffer EM, Nguyen D, Taub DD. The GHS-R blocker D-[Lys3] GHRP-6 serves as CCR5 chemokine receptor antagonist. Int J Med Sci. 2012; 9:51–58.
Article22. Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002; 159:1029–1037.
Article23. Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F, Ghe C, Isgaard J, Papotti M, Ghigo E, Muccioli G. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3',5'-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology. 2007; 148:512–529.
Article24. Kim MS, Yoon CY, Jang PG, Park YJ, Shin CS, Park HS, Ryu JW, Pak YK, Park JY, Lee KU, Kim SY, Lee HK, Kim YB, Park KS. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol Endocrinol. 2004; 18:2291–2301.
Article25. Chung H, Seo S, Moon M, Park S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J Endocrinol. 2008; 198:511–521.
Article26. Bonfili L, Cuccioloni M, Cecarini V, Mozzicafreddo M, Palermo FA, Cocci P, Angeletti M, Eleuteri AM. Ghrelin induces apoptosis in colon adenocarcinoma cells via proteasome inhibition and autophagy induction. Apoptosis. 2013; 18:1188–1200.
Article27. Conconi MT, Nico B, Guidolin D, Baiguera S, Spinazzi R, Rebuffat P, Malendowicz LK, Vacca A, Carraro G, Parnigotto PP, Nussdorfer GG, Ribatti D. Ghrelin inhibits FGF-2-mediated angiogenesis in vitro and in vivo. Peptides. 2004; 25:2179–2185.
Article28. Lam EW, Brosens JJ, Gomes AR, Koo CY. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer. 2013; 13:482–495.
Article29. Dong S, Kang S, Gu TL, Kardar S, Fu H, Lonial S, Khoury HJ, Khuri F, Chen J. 14-3-3 Integrates prosurvival signals mediated by the AKT and MAPK pathways in ZNF198-FGFR1-transformed hematopoietic cells. Blood. 2007; 110:360–369.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Chronic Restraint Stress on Body Weight, Food Intake, and Hypothalamic Gene Expressions in Mice
- Influences of DTC and zinc supplementation on the cellular response restoration in restrained mice
- Anti-Depressant Like Effect of Methyl Gallate Isolated from Acer barbinerve in Mice
- Repeated Short-term (2hx14d) Emotional Stress Induces Lasting Depression-like Behavior in Mice
- Apoptosis in Rat Thymus after Bolus Intramuscular Injection of 5-Fluorouracil