Immune Netw.
2016 Aug;16(4):233-241. 10.4110/in.2016.16.4.233.
Vanilloid Receptor 1 Agonists, Capsaicin and Resiniferatoxin, Enhance MHC Class I-restricted Viral Antigen Presentation in Virus-infected Dendritic Cells
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 28644, Korea. cklee@chungbuk.ac.kr
- KMID: 2349884
- DOI: http://doi.org/10.4110/in.2016.16.4.233
Abstract
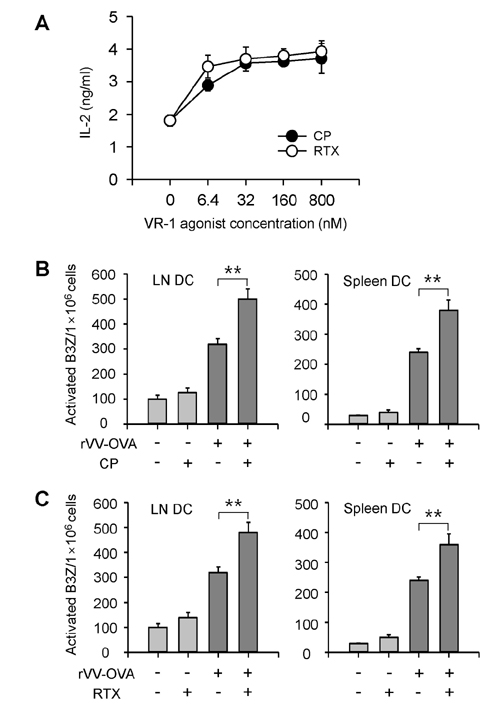
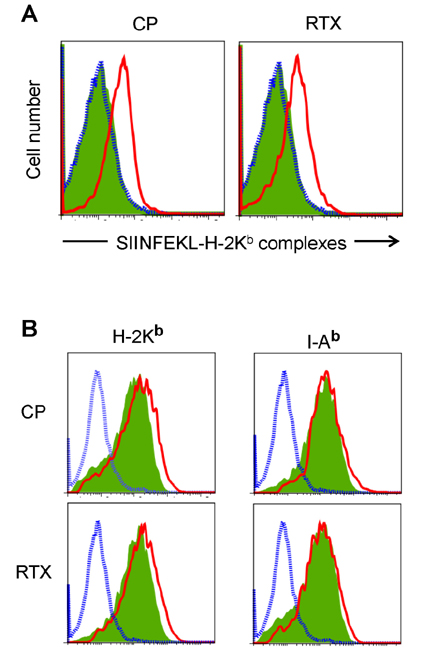
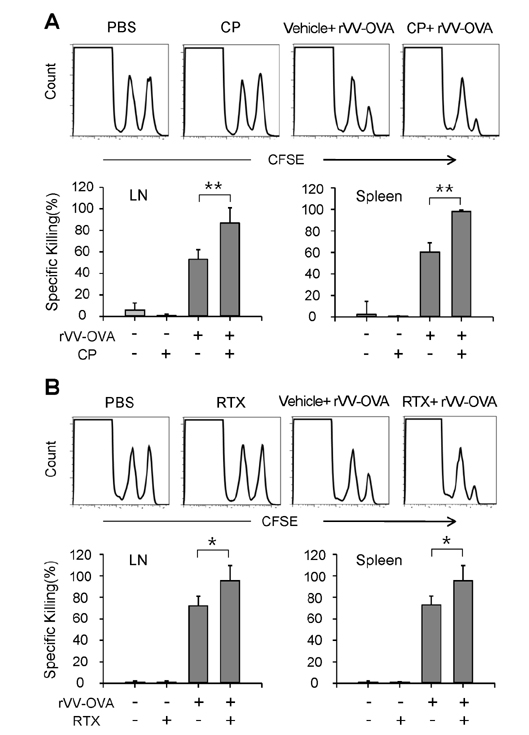
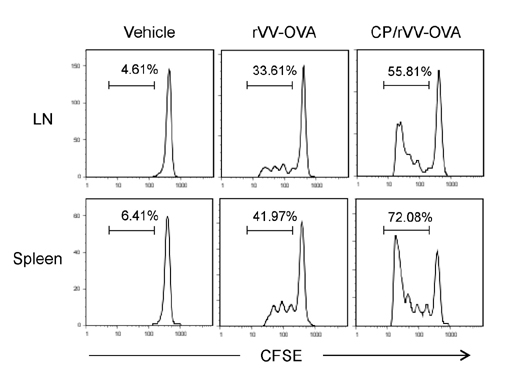
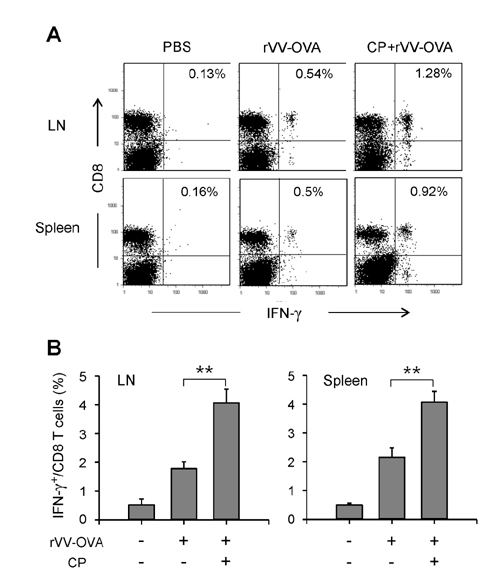
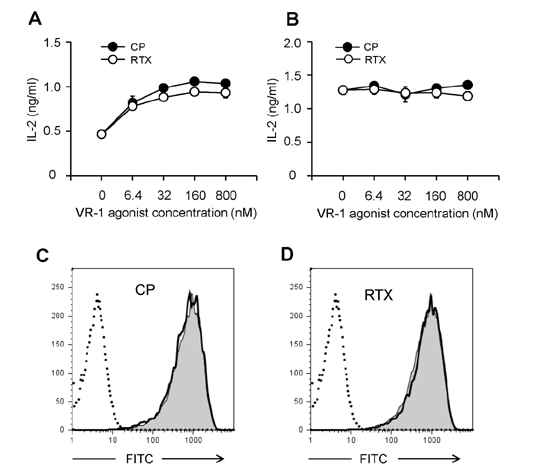
- DCs, like the sensory neurons, express vanilloid receptor 1 (VR1). Here we demonstrate that the VR1 agonists, capsaicin (CP) and resiniferatoxin (RTX), enhance antiviral CTL responses by increasing MHC class I-restricted viral antigen presentation in dendritic cells (DCs). Bone marrow-derived DCs (BM-DCs) were infected with a recombinant vaccinia virus (VV) expressing OVA (VV-OVA), and then treated with CP or RTX. Both CP and RTX increased MHC class I-restricted presentation of virus-encoded endogenous OVA in BM-DCs. Oral administration of CP or RTX significantly increased MHC class I-restricted OVA presentation by splenic and lymph node DCs in VV-OVA-infected mice, as assessed by directly measuring OVA peptide SIINFEKL-Kb complexes on the cell surface and by performing functional assays using OVA-specific CD8 T cells. Accordingly, oral administration of CP or RTX elicited potent OVA-specific CTL activity in VV-OVA-infected mice. The results from this study demonstrate that VR1 agonists enhance anti-viral CTL responses, as well as a neuro-immune connection in anti-viral immune responses.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Thapsigargin Increases IL-2 Production in T Cells at Nanomolar Concentrations
Ki-Hyang Kim, Sang-Hyun Kim, Ho-Hyun Jung, Jun-Hyeok Moon, Seong-Un Jeong, Kyeongae Yu, Chong-Kil Lee
Immune Netw. 2018;18(4):. doi: 10.4110/in.2018.18.e26.
Reference
-
1. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389:816–824.
Article2. Szallasi A, Blumberg PM. Vanilloid(Capsaicin)receptors and mechanisms. Pharmacol Rev. 1999; 51:159–212.3. Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007; 6:357–372.
Article4. Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetrei A, Dewachter I, van LF, Vennekens R, De RD, Nilius B, Voets T, Talavera K. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol. 2011; 21:316–321.
Article5. Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991; 43:143–201.6. Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989; 30:515–520.
Article7. Bley KR. Recent developments in transient receptor potential vanilloid receptor 1 agonist-based therapies. Expert Opin Investig Drugs. 2004; 13:1445–1456.
Article8. Nilsson G, Ahlstedt S. Altered lymphocyte proliferation of immunized rats after neurological manipulation with capsaicin. Int J Immunopharmacol. 1988; 10:747–751.
Article9. Gertsch J, Guttinger M, Sticher O, Heilmann J. Relative quantification of mRNA levels in Jurkat T cells with RT-real time-PCR (RT-rt-PCR): new possibilities for the screening of anti-inflammatory and cytotoxic compounds. Pharm Res. 2002; 19:1236–1243.10. Kim CS, Kawada T, Kim BS, Han IS, Choe SY, Kurata T, Yu R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-α degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003; 15:299–306.
Article11. Chen CW, Lee ST, Wu WT, Fu WM, Ho FM, Lin WW. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br J Pharmacol. 2003; 140:1077–1087.
Article12. Santoni G, Perfumi MC, Pompei P, Spreghini E, Lucciarini R, Martarelli D, Staffolani M, Piccoli M. Impairment of rat thymocyte differentiation and functions by neonatal capsaicin treatment is associated with induction of apoptosis. J Neuroimmunol. 2000; 104:37–46.
Article13. Kradin R, MacLean J, Duckett S, Schneeberger EE, Waeber C, Pinto C. Pulmonary response to inhaled antigen: neuroimmune interactions promote the recruitment of dendritic cells to the lung and the cellular immune response to inhaled antigen. Am J Pathol. 1997; 150:1735–1743.14. Basu S, Srivastava P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc Natl Acad Sci USA. 2005; 102:5120–5125.
Article15. Nevius E, Srivastava PK, Basu S. Oral ingestion of capsaicin, the pungent component of chili pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal Immunol. 2012; 5:76–86.
Article16. Mahmoud ME, Nikami H, Shiina T, Takewaki T, Shimizu Y. Capsaicin inhibits IFN-gamma-induced MHC class II expression by suppressing transcription of class II transactivator gene in murine peritoneal macrophages. Int Immunopharmacol. 2010; 10:86–90.
Article17. Karttunen , J , Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci USA. 1992; 89:6020–6024.
Article18. Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994; 153:4925–4933.19. Harding CV, Collins DS, Kanagawa O, Unanue ER. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991; 147:2860–2863.20. Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997; 6:715–726.
Article21. Lee YR, Yang IH, Lee YH, Im SA, Song S, Li H, Han K, Kim K, Eo SK, Lee CK. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005; 105:3951–3955.
Article22. Lee YH, Lee YR, Kim KH, Im SA, Song S, Lee MK, Kim Y, Hong JT, Kim K, Lee CK. Baccatin III, a synthetic precursor of taxol, enhances MHC-restricted antigen presentation in dendritic cells. Int Immunopharmacol. 2011; 11:985–991.
Article23. Lee YH, Lee YR, Im SA, Park SI, Kim KH, Gerelchuluun T, Song S, Kim K, Lee CK. Calcineurin inhibitors block MHC-restricted antigen presentation in vivo. J Immunol. 2007; 179:5711–5716.
Article24. Park CS, Im SA, Song S, Kim K, Lee CK. Identification of HLA-A2-restricted immunogenic peptides derived from a xenogenic porcine major histocompatibility complex. Xenotransplantation. 2014; 21:465–472.
Article25. Nilsson G, Alving K, Ahlstedt S. Effects on immune responses in the rats after neuromanipulation with capsaicin. Int Immunopharmacol. 1991; 13:21–26.
Article26. Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002; 20:621–667.
Article27. Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976; 143:1283–1288.
Article28. Harding CV. Phagocytic processing of antigens for presentation by MHC molecules. Trends Cell Biol. 1995; 5:105–109.
Article29. Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 2001; 1:126–134.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunomodulatory Effects of Hypocrellin A on MHC-restricted Antigen Processing
- Lectins Isolated from Mushroom Fomitella fraxinea Enhance MHC-restricted Exogenous Antigen Presentation
- Class Ii MHC and Viral Expression by Hematopoietic Progenitor Cells Infected with Friend Virus
- Evidence for Direct Inhibition of MHC-Restricted Antigen Processing by Dexamethasone
- Metformin Suppresses MHC-Restricted Antigen Presentation by Inhibiting Co-Stimulatory Factors and MHC Molecules in APCs