J Korean Ophthalmol Soc.
2016 Aug;57(8):1294-1298. 10.3341/jkos.2016.57.8.1294.
Spontaneous Regression of Lacrimal Sac Squamous Cell Carcinoma
- Affiliations
-
- 1Department of Ophthalmology, Yeungnam University College of Medicine, Daegu, Korea. sjh@med.yu.ac.kr
- KMID: 2349078
- DOI: http://doi.org/10.3341/jkos.2016.57.8.1294
Abstract
- PURPOSE
Spontaneous regression of squamous cell carcinoma is a very rare event. We report a case of primary squamous cell carcinoma in the lacrimal sac which showed spontaneous regression without any treatment.
CASE SUMMARY
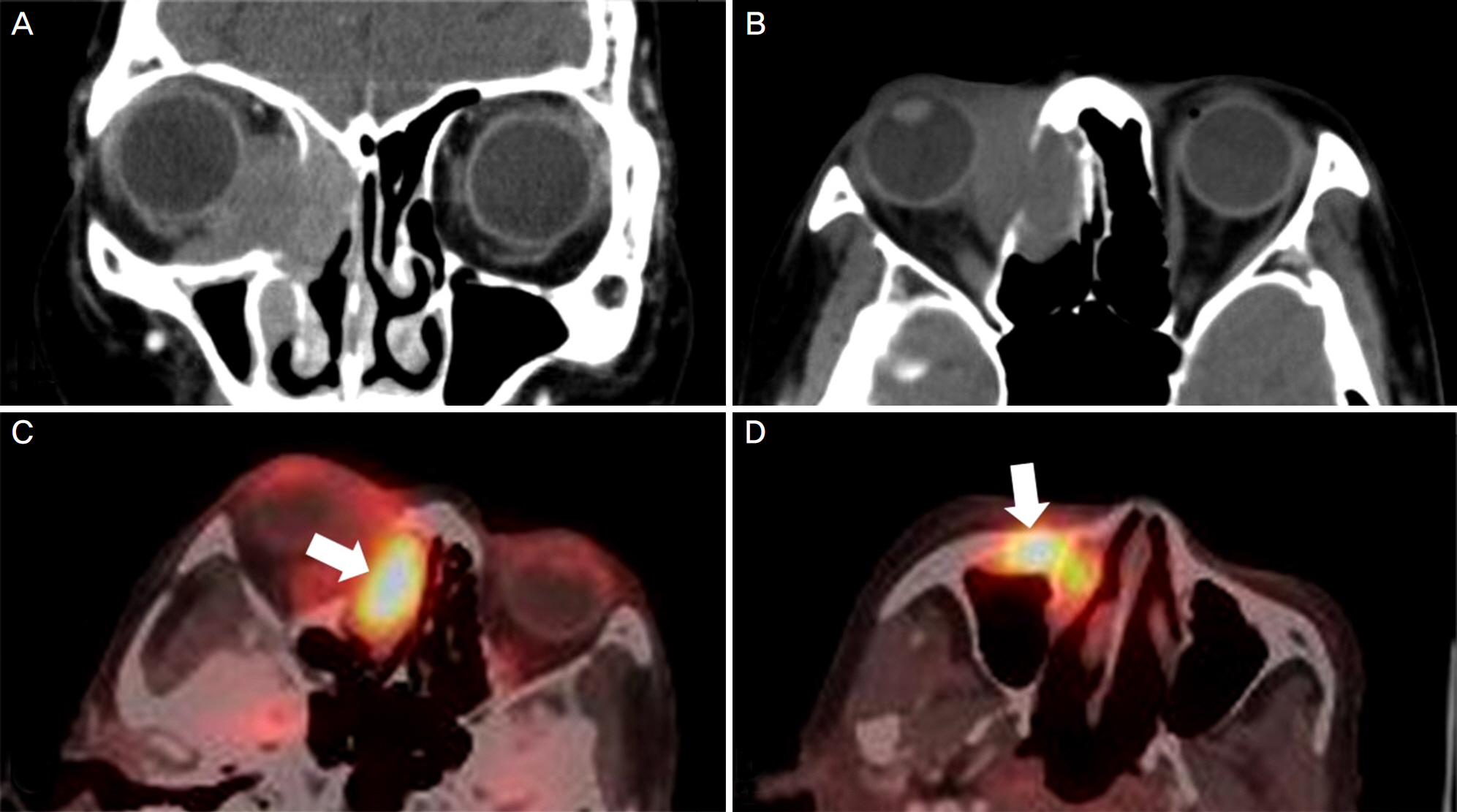
A 69-year-old woman presented with epiphora and ocular discharge from the right eye. Under the diagnosis of nasolacrimal duct obstruction, we performed dacryocystorhinostomy. Two years after the surgery, the patient presented again with severe epiphora and ocular pain accompanied by proptosis and adduction limitation in the right eye. Computed tomography demonstrated a mass occupying the right lacrimal sac and incisional biopsy showed poorly differentiated invasive squamous cell carcinoma. Additional treatment was recommended, but the patient denied any treatments. At 6 months after the biopsy, the medial orbital wall was partially destructed. On positron emission tomography/computed tomography, metastasis was suspected in the cervical, para-aortic, sub-carinal, and peri-esophageal lymph nodes. However, at 15 months after the biopsy, the orbital tumor had almost disappeared. On positron emission tomography/computed tomography, fluorodeoxyglucose uptake was reduced in all areas including the cervical and mediastinal lymph nodes.
CONCLUSIONS
This case exhibited a generally natural course of a malignant tumor, including medial orbital wall destruction and lymph node metastasis. However, the course then improved naturally without any treatment. The reason for the spontaneous regression of squamous cell carcinoma is still unclear but might be due to complex effects of one or several factors.
MeSH Terms
Figure
Reference
-
References
1. Cole WH, Everson TC. Spontaneous regression of cancer: abdominal report. Ann Surg. 1956; 144:366–83.2. Stoelben E, Koch M, Hanke S, et al. Spontaneous regression of hepatocellular carcinoma confirmed by surgical specimen: report of two cases and review of the literature. Langenbecks Arch Surg. 1998; 383:447–52.
Article3. Papac RJ. Spontaneous regression of cancer: possible mechanisms. In Vivo. 1998; 12:571–8.4. Oya R, Ikemura K. Spontaneous regression of recurrent squamous cell carcinoma of the tongue. Int J Clin Oncol. 2004; 9:339–42.
Article5. Ansai S, Manabe M. Possible spontaneous regression of a abdominal lesion of keratoacanthoma-like squamous cell carcinoma in a regional lymph node. J Dermatol. 2005; 32:899–903.6. Kurita M, Hirano K, Ebihara S, et al. Spontaneous regression of cervical lymph node metastasis in a patient with mesopharyngeal squamous cell carcinoma of the tongue: possible association abdominal apoptosis and tumor regression. Int J Clin Oncol. 2007; 12:448–54.7. de Andrade Sousa A, Lopes Rena R, Souza Silva G, et al. Spontaneous remission of a squamous cell carcinoma of the floor of the mouth. J Craniomaxillofac Surg. 2014; 42:1536–9.8. Foley C, Moran B, McMenamin M, et al. Spontaneous regression of cutaneous metastases of squamous cell carcinoma. QJM. 2014; 107:61–3.
Article9. Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981; 17:201–9.
Article10. Challis GB, Stam HJ. The spontaneous regression of cancer. A abdominal of cases from 1900 to 1987. Acta Oncol. 1990; 29:545–50.11. Bodey B. Spontaneous regression of neoplasms: new possibilities for immunotherapy. Expert Opin Biol Ther. 2002; 2:459–76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Squamous Cell Carcinoma of the Lacrimal Sac
- Squamous Cell Carcinoma in the Lacrimal Sac of Young Patient Who Had Conjunctival Squamous Papilloma
- A Case of Squamous Cell Carcinoma of the Lacrimal Sac
- Two Cases of Squamous Cell Carcinoma Arising from the Lid and Lacrimal Sac
- A Case of Squamous Cell Carcinoma Originating from the Lacrimal Sac