Korean Circ J.
2016 May;46(3):350-364. 10.4070/kcj.2016.46.3.350.
Two Distinct Responses of Left Ventricular End-Diastolic Pressure to Leg-Raise Exercise in Euvolemic Patients with Exertional Dyspnea
- Affiliations
-
- 1Department of Cardiology, Department of Internal Medicine, Guri Hospital, College of Medicine, Hanyang University, Guri, Korea. saint536@hanmail.net
- 2Division of Cardiology, Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 3Division of Cardiology, Department of Internal Medicine, Seoul SeungAe Hospital, Seoul, Korea.
- KMID: 2344448
- DOI: http://doi.org/10.4070/kcj.2016.46.3.350
Abstract
- BACKGROUND AND OBJECTIVES
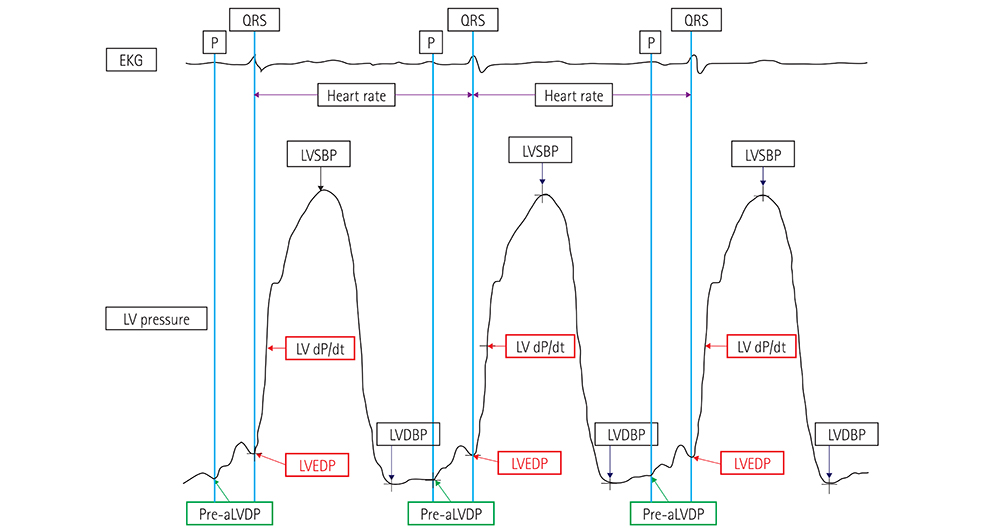
Few studies have invasively assessed diastolic functional reserve and serial changes in left ventricular hemodynamics in euvolemic patients with exertional dyspnea. In this study, sequential changes in left ventricular end-diastolic pressure (LVEDP) to leg-raise exercise were measured invasively in patients with early heart failure with preserved ejection fraction (HFpEF) to determine the association between these serial changes and echocardiographic results or clinical features.
SUBJECTS AND METHODS
During their hospital stay, 181 patients with early HFpEF underwent left cardiac catheterization, coronary angiography, and transthoracic echocardiography (TTE). Leg-raise exercise was performed in two stages: during cardiac catheterization and again during TTE.
RESULTS
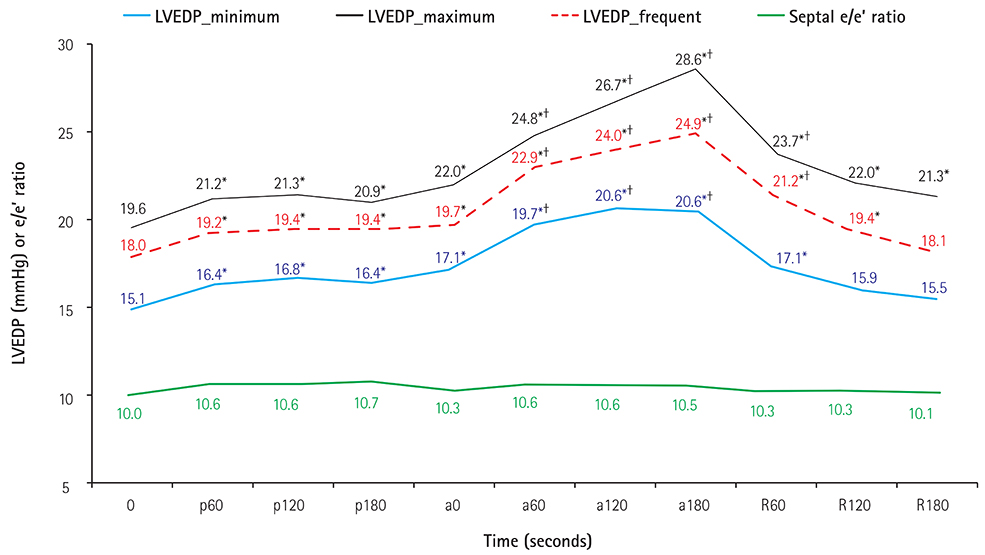
Compared with the initial values, all the invasively measured LVEDP values increased significantly during the leg-raise exercise, whereas the septal e/e' ratio remained unchanged. Active leg-raise led to increased LVEDP, which caused dyspnea. The severity of symptoms correlated with the level and extent of changes in LVEDP. At the end of active leg-raise, LVEDP decreased in 40 patients (22.1%), who were younger and had significantly lower e/e' ratios. On multivariate analysis to predict the response of LVEDP to active leg-raise, age and the septal e/e' ratio remained significant predictors.
CONCLUSION
Despite having similar LVEDP values at rest, patients may respond to exercise with different LVEDP levels and clinical manifestations, depending on their diastolic capacity. The leg-raise exercise in early HFpEF can elucidate individual diastolic profiles, and the LVEDP response to the leg-raise test may serve as a useful criterion in stratifying patients with early HFpEF with respect to functional reserve.
MeSH Terms
Figure
Reference
-
1. Ha JW, Choi D, Park S, et al. Left ventricular diastolic functional reserve during exercise in patients with impaired myocardial relaxation at rest. Heart. 2009; 95:399–404.2. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010; 3:588–595.3. Lam CS. Heart failure with preserved ejection fraction: invasive solution to diagnostic confusion? J Am Coll Cardiol. 2010; 55:1711–1712.4. Dorfs S, Zeh W, Hochholzer W, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014; 35:3103–3112.5. Penicka M, Bartunek J, Trakalova H, et al. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure-volume loop analysis. J Am Coll Cardiol. 2010; 55:1701–1710.6. Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007; 28:2539–2550.7. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011; 32:670–679.8. Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011; 97:964–969.9. Chattopadhyay S, Alamgir MF, Nikitin NP, Rigby AS, Clark AL, Cleland JG. Lack of diastolic reserve in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2010; 3:35–43.10. Choi EY, Shim CY, Kim SA, et al. Passive leg-raise is helpful to identify impaired diastolic functional reserve during exercise in patients with abnormal myocardial relaxation. J Am Soc Echocardiogr. 2010; 23:523–530.11. Ishizu T, Seo Y, Kawano S, Watanabe S, Ishimitsu T, Aonuma K. Stratification of impaired relaxation filling patterns by passive leg lifting in patients with preserved left ventricular ejection fraction. Eur J Heart Fail. 2008; 10:1094–1101.12. Obokata M, Negishi K, Kurosawa K, et al. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013; 6:749–758.13. Kerner W, Brückel J. German Diabetes Association. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014; 122:384–386.14. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004; 44:720–732.15. Previtali M, Chieffo E, Ferrario M, Klersy C. Is mitral e/e' ratio a reliable predictor of left ventricular diastolic pressures in patients without heart failure? Eur Heart J Cardiovasc Imaging. 2012; 13:588–595.16. Takeichi Y, Yokota M, Iwase M, et al. Biphasic changes in left ventricular end-diastolic pressure during dynamic exercise in patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2001; 38:335–343.17. Hittinger L, Patrick T, Ihara T, et al. Exercise induces cardiac dysfunction in both moderate, compensated and severe hypertrophy. Circulation. 1994; 89:2219–2231.18. Erdei T, Aakhus S, Marino P, Paulus WJ, Smiseth OA, Fraser AG. Pathophysiological rationale and diagnostic targets for diastolic stress testing. Heart. 2015; 101:1355–1360.19. Edelmann F, Gelbrich G, Düngen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011; 58:1780–1791.20. Maier LS, Layug B, Karwatowska-Prokopczuk E, et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Failure. 2013; 1:115–122.21. Ingle L, Cleland JG, Clark AL. Perception of symptoms is out of proportion to cardiac pathology in patients with "diastolic heart failure". Heart. 2008; 94:748–753.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Contractile and Relaxing Functions of the Left Ventricle and Its Responses to Nitroprusside in Hypertensive Hypertrophic Heart Disease
- Evaluation of Left Ventricular Function by Dynamic Exercise Echocardiography in Normal Subjects
- Relationship between Exercise-induced Blood Pressure Response and Left Ventricular Hypertrophy in Patients with Hypertension
- Assessment of Left Ventricular Diastolic Pressures with Pulmonary Venous Flow and Transmitral Inflow by Doppler Echocardiography
- Echocardiographic Evaluation of Left Ventricle before and after Maximum Exercise in Track Athletes