Korean Circ J.
2016 Jul;46(4):562-568. 10.4070/kcj.2016.46.4.562.
Influence of Thromboxane Aâ‚‚ on the Regulation of Adenosine Triphosphate-Sensitive Potassium Channels in Mouse Ventricular Myocytes
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Chonnam National University Medical School, Gwangju, Korea.
- 2Department of Pediatrics, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 4Department of Pharmacology, Chonnam National University Medical School, Gwangju, Korea. jkkim57@jnu.ac.kr
- KMID: 2344433
- DOI: http://doi.org/10.4070/kcj.2016.46.4.562
Abstract
- BACKGROUND AND OBJECTIVES
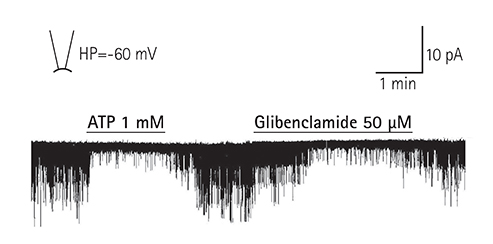
Adenosine triphosphate (ATP)-sensitive potassium (K(ATP)) channels play an important role in myocardial protection. We examined the effects of thromboxane Aâ‚‚ on the regulation of K(ATP) channel activity in single ventricular myocytes.
SUBJECTS AND METHODS
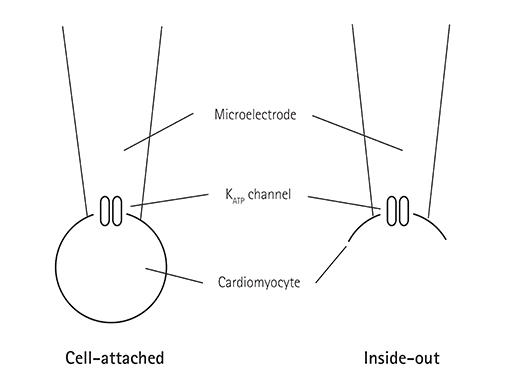
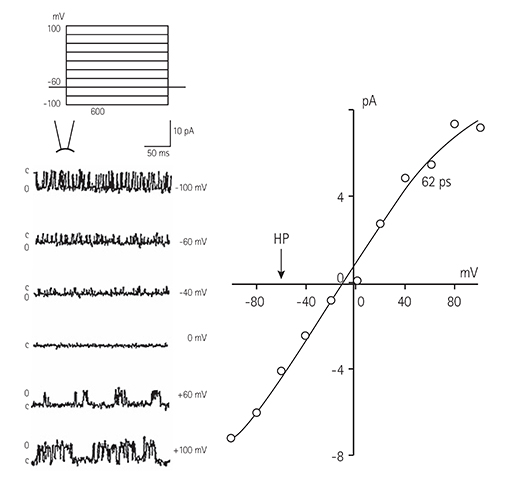
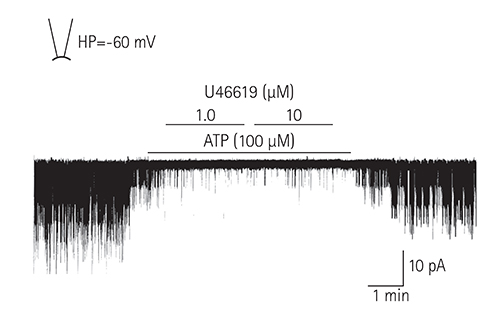
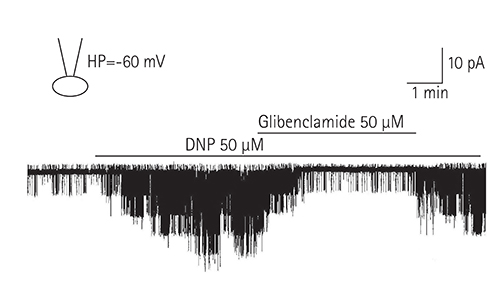
Single ventricular myocytes were isolated from the hearts of adult Institute of Cancer Research (ICR) mice by enzymatic digestion. Single channel activity was recorded by excised inside-out and cell-attached patch clamp configurations at -60 mV holding potential during the perfusion of an ATP-free K-5 solution.
RESULTS
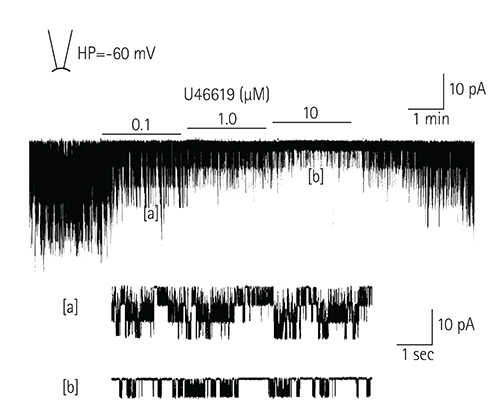
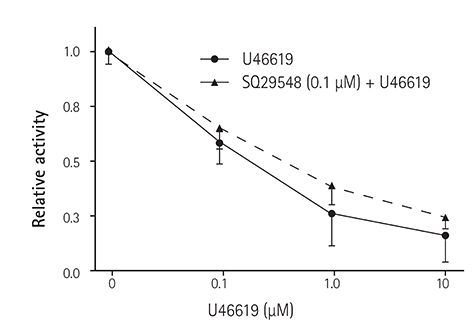
In the excised inside-out patches, the thromboxane Aâ‚‚ analog, U46619, decreased the K(ATP) channel activity in a dose-dependent manner; however, the thromboxane Aâ‚‚ receptor antagonist, SQ29548, did not significantly attenuate the inhibitory effect of U46619. In the cell-attached patches, U46619 inhibited dinitrophenol (DNP)-induced K(ATP) channel activity in a dose-dependent manner, and SQ29548 attenuated the inhibitory effects of U46619 on DNP-induced K(ATP) channel activity.
CONCLUSION
Thromboxane Aâ‚‚ may inhibit K(ATP) channel activity, and may have a harmful effect on ischemic myocardium.
Keyword
MeSH Terms
-
15-Hydroxy-11 alpha,9 alpha-(epoxymethano)prosta-5,13-dienoic Acid
Adenosine Triphosphate
Adenosine*
Adult
Animals
Digestion
Heart
Humans
KATP Channels
Mice*
Muscle Cells*
Myocardium
Perfusion
Potassium Channels*
Potassium*
15-Hydroxy-11 alpha,9 alpha-(epoxymethano)prosta-5,13-dienoic Acid
Adenosine
Adenosine Triphosphate
KATP Channels
Potassium
Potassium Channels
Figure
Reference
-
1. Neher E, Sakmann B. The patch clamp technique. Sci Am. 1992; 266:44–51.2. Ackerman MJ, Clapham DE. Ion channels--basic science and clinical disease. N Engl J Med. 1997; 336:1575–1586.3. Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983; 305:147–148.4. Trube G, Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984; 401:178–184.5. Kléber AG. Extracellular potassium accumulation in acute myocardial ischemia. J Mol Cell Cardiol. 1984; 16:389–394.6. Hill JL, Gettes LS. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation. 1980; 61:768–778.7. Coetzee WA. ATP-sensitive potassium channels and myocardial ischemia: why do they open? Cardiovasc Drugs Ther. 1992; 6:201–208.8. Antzelevitch C, Di Diego JM. Role of K+ channel activators in cardiac electrophysiology and arrhythmias. Circulation. 1992; 85:1627–1629.9. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981; 391:85–100.10. Kirsch GE, Codina J, Birnbaumer L, Brown AM. Coupling of ATP-sensitive K+ channels to A1 receptors by G proteins in rat ventricular myocytes. Am J Physiol. 1990; 259(3 Pt 2):H820–H826.11. Quayle JM, Standen NB. KATP channels in vascular smooth muscle. Cardiovasc Res. 1994; 28:797–804.12. Ju JM, Nah SY, Kim JH. Effect of prostaglandins D2, E2 and I2 on the regulation of KATP channel activity in rat cardiac myocytes. Korean J Physiol Pharmacol. 1999; 3:507–512.13. Ju JM, Shin DH, Jeong HS, Park HW, Cho JG, Kim JH. Effects of endothelium-derived relaxing factors on the regulation of ATP-sensitive potassium channel activity in cardiac myocytes. Korean Circ J. 2003; 33:420–430.14. Kubo M, Nakaya Y, Matsuoka S, Saito K, Kuroda Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle cells. Circ Res. 1994; 74:471–476.15. Furukawa K, Itoh T, Kajiwara M, et al. Vasodilating actions of 2-nicotinamidoethyl nitrate on porcine and guinea-pig coronary arteries. J Pharmacol Exp Ther. 1981; 218:248–259.16. Quast U. Potassium channel openers: pharmacological and clinical aspects. Fundam Clin Pharmacol. 1992; 6:279–293.17. Schmid-Antomarchi H, De Weille J, Fosset M, Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987; 262:15840–15844.18. Bouchard JF, Dumont E, Lamontagne D. Evidence that prostaglandins I2, E2, and D2 may activate ATP-sensitive potassium channels in the isolated rat heart. Cardiovasc Res. 1994; 28:901–905.19. Jackson WF, König A, Dambacher T, Busse R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol. 1993; 264(1 Pt 2):H238–H243.20. Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Czeta. Circ Res. 2003; 93:656–663.21. Li QJ, Janssen LJ. Membrane currents in canine bronchial artery and their regulation by excitatory agonists. Am J Physiol Lung Cell Mol Physiol. 2002; 282:L1358–L1365.22. Scornik FS, Toro L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am J Physiol. 1992; 262(3 Pt 1):C708–C713.23. Xiao CY, Hara A, Yuhki K, et al. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 2001; 104:2210–2215.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of micron-Opioid Agonist on ATP-sensitive Potassium Channel Activity in Isolated Ventricular Cardiomyocytes
- Effects of Endothelium-derived Relaxing Factors on the Regulation of ATP-sensitive Potassium Channel Activity in Cardiac Myocytes
- The effects of intracellular monocarboxylates on the ATP-sensitive potassium channels in rabbit ventricular myocytes
- Role of Plasmalogen-derived Lysolipids onthe Pathophysiology of Myocardial Ischemia
- The Effect of Trifluoroacetic Acid, a Metabolite of Isoflurane on the ATP-sensitive Potassium Channel in Rabbit Ventricular Myocytes