J Vet Sci.
2015 Sep;16(3):373-380. 10.4142/jvs.2015.16.3.373.
Rapamycin treatment during in vitro maturation of oocytes improves embryonic development after parthenogenesis and somatic cell nuclear transfer in pigs
- Affiliations
-
- 1College of Veterinary Medicine, Kangwon National University, Chuncheon 200-701, Korea. eslee@kangwon.ac.kr
- 2College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- 3Wide River Institute of Immunology, Seoul National University, Hongcheon 250-812, Korea.
- 4Division of Applied Animal Science,Department of Animal Biotechology, College of Animal Life Science, Kangwon National University, Chuncheon 200-701, Korea.
- 5College of Veterinary Medicine, Chungbuk National University, Cheongju 362-763, Korea.
- 6Institute of Veterinary Science, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 2344311
- DOI: http://doi.org/10.4142/jvs.2015.16.3.373
Abstract
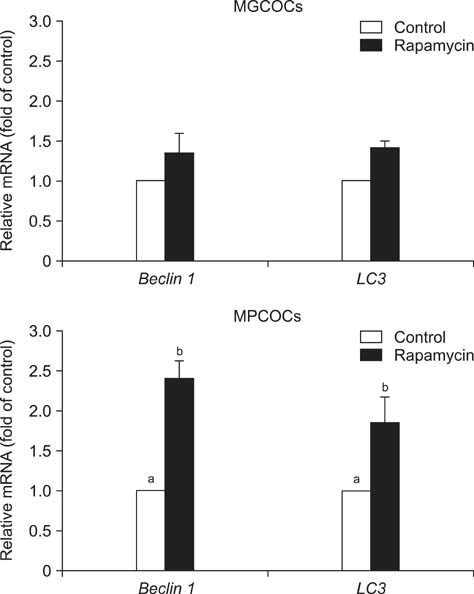
- This study was conducted to investigate the effects of rapamycin treatment during in vitro maturation (IVM) on oocyte maturation and embryonic development after parthenogenetic activation (PA) and somatic cell nuclear transfer (SCNT) in pigs. Morphologically good (MGCOCs) and poor oocytes (MPCOCs) were untreated or treated with 1 nM rapamycin during 0-22 h, 22-42 h, or 0-42 h of IVM. Rapamycin had no significant effects on nuclear maturation and blastocyst formation after PA of MGCOCs. Blastocyst formation after PA was significantly increased by rapamycin treatment during 22-42 h and 0-42 h (46.6% and 46.5%, respectively) relative to the control (33.3%) and 0-22 h groups (38.6%) in MPCOCs. In SCNT, blastocyst formation tended to increase in MPCOCs treated with rapamycin during 0-42 h of IVM relative to untreated oocytes (20.3% vs. 14.3%, 0.05 < p < 0.1), while no improvement was observed in MGCOCs. Gene expression analysis revealed that transcript abundance of Beclin 1 and microtubule-associated protein 1 light chain 3 mRNAs was significantly increased in MPCOCs by rapamycin relative to the control. Our results demonstrated that autophagy induction by rapamycin during IVM improved developmental competence of oocytes derived from MPCOCs.
MeSH Terms
Figure
Reference
-
1. Bavister BD, Leibfried ML, Lieberman G. Development of preimplantation embryos of the golden hamster in a defined culture medium. Biol Reprod. 1983; 28:235–247.
Article2. Bing YZ, Hirao Y, Iga K, Che LM, Takenouchi N, Kuwayama M, Fuchimoto D, Rodriguez-Martinez H, Nagai T. In vitro maturation and glutathione synthesis of porcine oocytes in the presence or absence of cysteamine under different oxygen tensions: role of cumulus cells. Reprod Fertil Dev. 2002; 14:125–131.
Article3. de Matos DG, Furnus CC, Moses DF. Glutathione synthesis during in vitro maturation of bovine oocytes: role of cumulus cells. Biol Reprod. 1997; 57:1420–1425.
Article4. Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod. 2005; 73:351–357.
Article5. Fatehi AN, Roelen BA, Colenbrander B, Schoevers EJ, Gadella BM, Beverst MM, van den Hurk R. Presence of cumulus cells during in vitro fertilization protects the bovine oocyte against oxidative stress and improves first cleavage but does not affect further development. Zygote. 2005; 13:177–185.
Article6. Ferraro E, Cecconi F. Autophagic and apoptotic response to stress signals in mammalian cells. Arch Biochem Biophys. 2007; 462:210–219.
Article7. Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010; 285:14071–14077.
Article8. Funahashi H, Kim NH, Stumpf TT, Cantley TC, Day BN. Presence of organic osmolytes in maturation medium enhances cytoplasmic maturation of porcine oocytes. Biol Reprod. 1996; 54:1412–1419.
Article9. Hashimoto N, Kishimoto T. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Dev Biol. 1998; 126:242–252.
Article10. Ju S, Rui R. Effects of cumulus cells on in vitro maturation of oocytes and development of cloned embryos in the pig. Reprod Domest Anim. 2012; 47:521–529.
Article11. Kim J, You J, Hyun SH, Lee G, Lim J, Lee E. Developmental competence of morphologically poor oocytes in relation to follicular size and oocyte diameter in the pig. Mol Reprod Dev. 2010; 77:330–339.
Article12. Kim KS, Minami N, Yamada M, Utsumi K. Follicular cells affect the fertilizability and developmental competency of bovine oocytes in vitro. Reprod Fertil Dev. 1997; 9:763–766.
Article13. Kogasaka Y, Hoshino Y, Hiradate Y, Tanemura K, Sato E. Distribution and association of mTOR with its cofactors, raptor and rictor, in cumulus cells and oocytes during meiotic maturation in mice. Mol Reprod Dev. 2013; 80:334–348.
Article14. Krisher RL. The effect of oocyte quality on development. J Anim Sci. 2004; 82:Suppl. E14–E23.15. Kubelka M, Anger M, Kalous J, Schultz RM, Motlík J. Chromosome condensation in pig oocytes: lack of a requirement for either cdc2 kinase or MAP kinase activity. Mol Reprod Dev. 2002; 63:110–118.
Article16. Kubelka M, Rimkeviĉová Z, Guerrier P, Motlík J. Inhibition of protein synthesis affects histone H1 kinase, but not chromosome condensation activity, during the first meiotic division of pig oocytes. Mol Reprod Dev. 1995; 41:63–69.
Article17. Lee J, You J, Lee GS, Hyun SH, Lee E. Pig oocytes with a large perivitelline space matured in vitro show greater developmental competence after parthenogenesis and somatic cell nuclear transfer. Mol Reprod Dev. 2013; 80:753–762.
Article18. Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999; 402:672–676.
Article19. Luciano AM, Lodde V, Beretta MS, Colleoni S, Lauria A, Modina S. Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus-oocyte complexes: role of cumulus cells, cyclic adenosine 3',5' -monophosphate, and glutathione. Mol Reprod Dev. 2005; 71:389–397.
Article20. Maedomari N, Kikuchi K, Ozawa M, Noguchi J, Kaneko H, Ohnuma K, Nakai M, Shino M, Nagai T, Kashiwazaki N. Cytoplasmic glutathione regulated by cumulus cells during porcine oocyte maturation affects fertilization and embryonic development in vitro. Theriogenology. 2007; 67:983–993.
Article21. Othman EQG, Kaur G, Mutee AF, Muhammad TST, Tan ML. Immunohistochemical expression of MAP1LC3A and MAP1LC3B protein in breast carcinoma tissues. J Clin Lab Anal. 2009; 23:249–258.
Article22. Pan T, Rawal P, Wu Y, Xie W, Jankovic J, Le W. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience. 2009; 164:541–551.
Article23. Sakatani M, Suda I, Oki T, Kobayashi S, Kobayashi S, Takahashi M. Effects of purple sweet potato anthocyanins on development and intracellular redox status of bovine preimplantation embryos exposed to heat shock. J Reprod Dev. 2007; 53:605–614.
Article24. Salmen JJ, Skufca F, Matt A, Gushansky G, Mason A, Gardiner CS. Role of glutathione in reproductive tract secretions on mouse preimplantation embryo development. Biol Reprod. 2005; 73:308–314.
Article25. Santiquet NW, Develle Y, Laroche A, Robert C, Richard FJ. Regulation of gap-junctional communication between cumulus cells during in vitro maturation in swine, a gap-FRAP study. Biol Reprod. 2012; 87:46.26. Song BS, Yoon SB, Kim JS, Sim BW, Kim YH, Cha JJ, Choi SA, Min HK, Lee Y, Huh JW, Lee SR, Kim SH, Koo DB, Choo YK, Kim HM, Kim SU, Chang KT. Induction of autophagy promotes preattachment development of bovine embryos by reducing endoplasmic reticulum stress. Biol Reprod. 2012; 87:8.27. Song BS, Kim JS, Kim YH, Sim BW, Yoon SB, Cha JJ, Choi SA, Yang HJ, Mun SE, Park YH, Jeong KJ, Huh JW, Lee SR, Kim SH, Kim SU, Chang KT. Induction of autophagy during in vitro maturation improves the nuclear and cytoplasmic maturation of porcine oocytes. Reprod Fertil Dev. 2014; 26:974–981.
Article28. Song K, Hyun SH, Shin T, Lee E. Post-activation treatment with demecolcine improves development of somatic cell nuclear transfer embryos in pigs by modifying the remodeling of donor nuclei. Mol Reprod Dev. 2009; 76:611–619.
Article29. Su J, Wang Y, Li R, Peng H, Hua S, Li Q, Quan F, Guo Z, Zhang Y. Oocytes selected using BCB staining enhance nuclear reprogramming and the in vivo development of SCNT embryos in cattle. PLoS One. 2012; 7:e36181.30. Sully K, Akinduro O, Philpott MP, Naeem AS, Harwood CA, Reeve VE, O'Shaughnessy RF, Byrne C. The mTOR inhibitor rapamycin opposes carcinogenic changes to epidermal Akt1/PKBXMLLink_XYZ isoform signaling. Oncogene. 2013; 32:3254–3262.
Article31. Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod. 2000; 63:805–810.
Article32. Tirado OM, Mateo-Lozano S, Notario V. Rapamycin induces apoptosis of JN-DSRCT-1 cells by increasing the Bax : Bcl-xL ratio through concurrent mechanisms dependent and independent of its mTOR inhibitory activity. Oncogene. 2005; 24:3348–3357.
Article33. Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993; 333:169–174.
Article34. Tsukamoto S, Kuma A, Mizushima N. The role of autophagy during the oocyte-to-embryo transition. Autophagy. 2008; 4:1076–1078.
Article35. Walker SC, Shin T, Zaunbrecher GM, Romano JE, Johnson GA, Bazer FW, Piedrahita JA. A highly efficient method for porcine cloning by nuclear transfer using in vitro-matured oocytes. Cloning Stem Cells. 2002; 4:105–112.
Article36. Wongsrikeao P, Kaneshige Y, Ooki R, Taniguchi M, Agung B, Nii M, Otoi T. Effect of the removal of cumulus cells on the nuclear maturation, fertilization and development of porcine oocytes. Reprod Domest Anim. 2005; 40:166–170.
Article37. Xu YN, Shen XH, Lee SE, Kwon JS, Kim DJ, Heo YT, Cui XS, Kim NH. Autophagy influences maternal mRNA degradation and apoptosis in porcine parthenotes developing in vitro. J Reprod Dev. 2012; 58:576–584.
Article38. Yoshida M, Ishigaki K, Nagai T, Chikyu M, Pursel VG. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol Reprod. 1993; 49:89–94.
Article39. You J, Lee J, Hyun SH, Lee E. L-carnitine treatment during oocyte maturation improves in vitro development of cloned pig embryos by influencing intracellular glutathione synthesis and embryonic gene expression. Theriogenology. 2012; 78:235–243.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oocyte maturation under a biophoton generator improves preimplantation development of pig embryos derived by parthenogenesis and somatic cell nuclear transfer
- In vitro maturation using αMEM with reduced NaCl enhances maturation and developmental competence of pig oocytes after somatic cell nuclear transfer
- Modification of maturation condition improves oocyte maturation and in vitro development of somatic cell nuclear transfer pig embryos
- Dynamic analysis of Ca2+ level during bovine oocytes maturation and early embryonic development
- Various macromolecules in in vitro growth medium influence growth, maturation, and parthenogenetic development of pig oocytes derived from small antral follicles