Korean J Radiol.
2015 Dec;16(6):1303-1312. 10.3348/kjr.2015.16.6.1303.
Accuracy of Diffusion Tensor Imaging for Diagnosing Cervical Spondylotic Myelopathy in Patients Showing Spinal Cord Compression
- Affiliations
-
- 1Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul 06273, Korea. agn70@yuhs.ac
- 2Department of Radiology, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Korea.
- 3Department of Radiology, Utah Center for Advanced Imaging Research, University of Utah, Salt Lake City, UT 84112, USA.
- 4Siemens Healthcare, Seoul 03737, Korea.
- 5Department of Rehabilitation Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul 06273, Korea.
- KMID: 2344285
- DOI: http://doi.org/10.3348/kjr.2015.16.6.1303
Abstract
OBJECTIVE
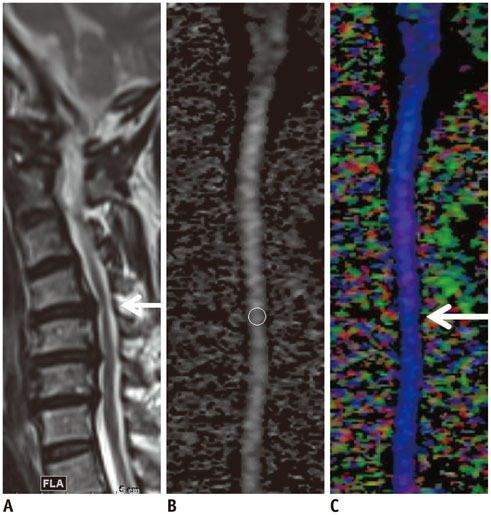
To assess the performance of diffusion tensor imaging (DTI) for the diagnosis of cervical spondylotic myelopathy (CSM) in patients with deformed spinal cord but otherwise unremarkable conventional magnetic resonance imaging (MRI) findings.
MATERIALS AND METHODS
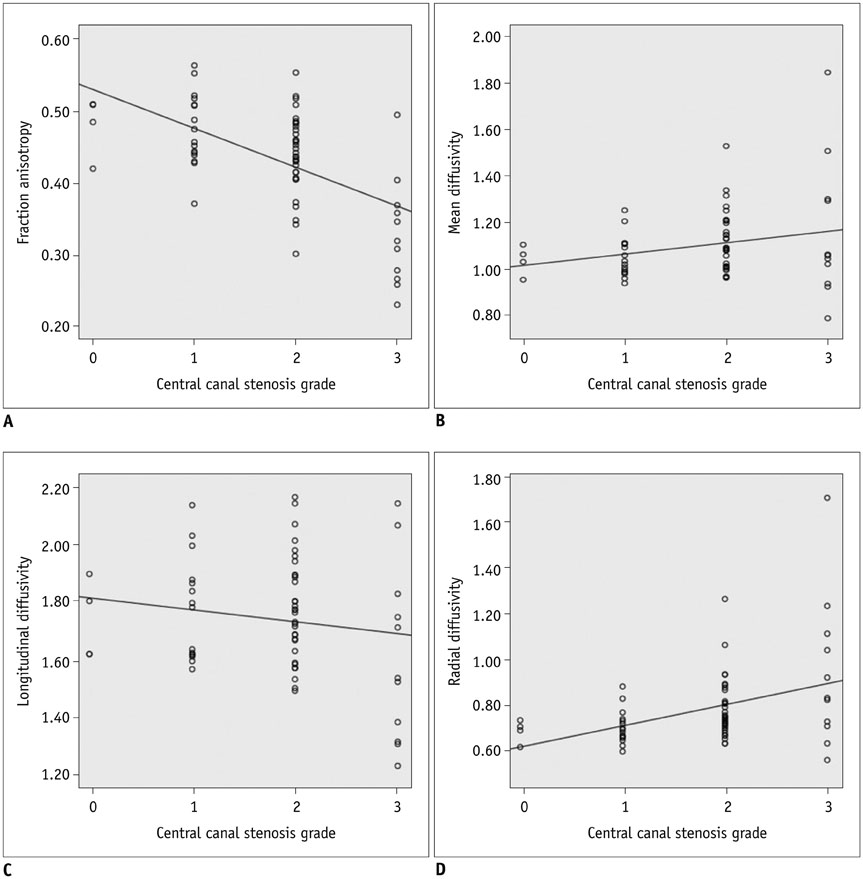
A total of 33 patients who underwent MRI of the cervical spine including DTI using two-dimensional single-shot interleaved multi-section inner volume diffusion-weighted echo-planar imaging and whose spinal cords were deformed but showed no signal changes on conventional MRI were the subjects of this study. Mean diffusivity (MD), longitudinal diffusivity (LD), radial diffusivity (RD), and fractional anisotropy (FA) were measured at the most stenotic level. The calculated performance of MD, FA, MD∩FA (considered positive when both the MD and FA results were positive), LD∩FA (considered positive when both the LD and FA results were positive), and RD∩FA (considered positive when both the RD and FA results were positive) in diagnosing CSM were compared with each other based on the estimated cut-off values of MD, LD, RD, and FA from receiver operating characteristic curve analysis with the clinical diagnosis of CSM from medical records as the reference standard.
RESULTS
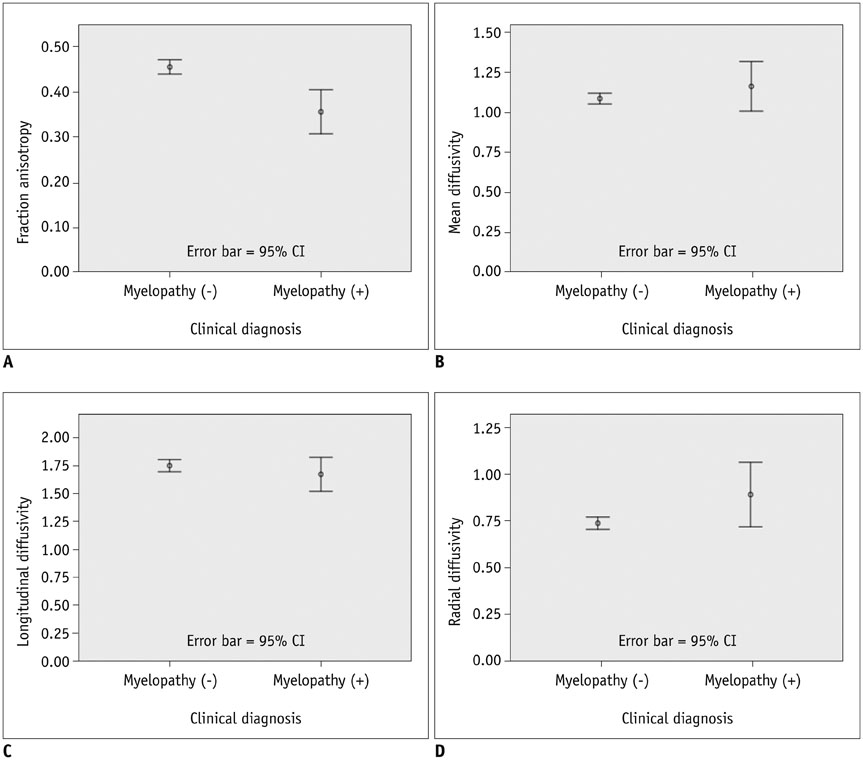
The MD, LD, and RD cut-off values were 1.079 × 10⻳, 1.719 × 10⻳, and 0.749 × 10⻳ mm²/sec, respectively, and that of FA was 0.475. Sensitivity, specificity, positive predictive value and negative predictive value were: 100 (4/4), 44.8 (13/29), 20 (4/20), and 100 (13/13) for MD; 100 (4/4), 27.6 (8/29), 16 (4/25), and 100 (8/8) for FA; 100 (4/4), 58.6 (17/29), 25 (4/16), and 100 (17/17) for MD∩FA; 100 (4/4), 68.9 (20/29), 30.8 (4/13), and 100 (20/20) for LD∩FA; and 75 (3/4), 68.9 (20/29), 25 (3/12), and 95.2 (20/21) for RD∩FA in percentage value. Diagnostic performance comparisons revealed significant differences only in specificity between FA and MD∩FA (p = 0.003), FA and LD∩FA (p < 0.001), FA and RD∩FA (p < 0.001), MD and LD∩FA (p = 0.024) and MD and RD∩FA (p = 0.024).
CONCLUSION
Fractional anisotropy combined with MD, RD, or LD is expected to be more useful than FA and MD for diagnosing CSM in patients who show deformed spinal cords without signal changes on MRI.
Keyword
MeSH Terms
Figure
Reference
-
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006; 6:6 Suppl. 190S–197S.2. Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007; 60(1 Supp1 1):S35–S41.3. Matsuda Y, Miyazaki K, Tada K, Yasuda A, Nakayama T, Murakami H, et al. Increased MR signal intensity due to cervical myelopathy. Analysis of 29 surgical cases. J Neurosurg. 1991; 74:887–889.4. Matsumoto M, Toyama Y, Ishikawa M, Chiba K, Suzuki N, Fujimura Y. Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy Does it predict the outcome of conservative treatment? Spine (Phila Pa 1976). 2000; 25:677–682.5. Takahashi M, Yamashita Y, Sakamoto Y, Kojima R. Chronic cervical cord compression: clinical significance of increased signal intensity on MR images. Radiology. 1989; 173:219–224.6. al-Mefty O, Harkey HL, Marawi I, Haines DE, Peeler DF, Wilner HI, et al. Experimental chronic compressive cervical myelopathy. J Neurosurg. 1993; 79:550–561.7. Bednarik J, Kadanka Z, Dusek L, Kerkovsky M, Vohanka S, Novotny O, et al. Presymptomatic spondylotic cervical myelopathy: an updated predictive model. Eur Spine J. 2008; 17:421–431.8. Bednarik J, Kadanka Z, Dusek L, Novotny O, Surelova D, Urbanek I, et al. Presymptomatic spondylotic cervical cord compression. Spine (Phila Pa 1976). 2004; 29:2260–2269.9. Kadanka Z, Kerkovsky M, Bednarik J, Jarkovsky J. Cross-sectional transverse area and hyperintensities on magnetic resonance imaging in relation to the clinical picture in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2007; 32:2573–2277.10. Demir A, Ries M, Moonen CT, Vital JM, Dehais J, Arne P, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology. 2003; 229:37–43.11. Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005; 26:1587–1594.12. Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging. 2005; 22:38–43.13. Hori M, Okubo T, Aoki S, Kumagai H, Araki T. Line scan diffusion tensor MRI at low magnetic field strength: feasibility study of cervical spondylotic myelopathy in an early clinical stage. J Magn Reson Imaging. 2006; 23:183–188.14. Kim TH, Zollinger L, Shi XF, Kim SE, Rose J, Patel AA, et al. Quantification of diffusivities of the human cervical spinal cord using a 2D single-shot interleaved multisection inner volume diffusion-weighted echo-planar imaging technique. AJNR Am J Neuroradiol. 2010; 31:682–687.15. Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011; 21:426–433.16. Kara B, Celik A, Karadereler S, Ulusoy L, Ganiyusufoglu K, Onat L, et al. The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology. 2011; 53:609–616.17. Lee JW, Kim JH, Park JB, Park KW, Yeom JS, Lee GY, et al. Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: preliminary results. Skeletal Radiol. 2011; 40:1543–1551.18. Kerkovský M, Bednarík J, Dušek L, Sprláková-Puková A, Urbánek I, Mechl M, et al. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976). 2012; 37:48–56.19. Gao SJ, Yuan X, Jiang XY, Liu XX, Liu XP, Wang YF, et al. Correlation study of 3T-MR-DTI measurements and clinical symptoms of cervical spondylotic myelopathy. Eur J Radiol. 2013; 82:1940–1945.20. Ellingson BM, Salamon N, Grinstead JW, Holly LT. Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J. 2014; 14:2589–2597.21. Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002; 17:1429–1436.22. Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003; 20:1714–1722.23. Ellingson BM, Kurpad SN, Schmit BD. Functional correlates of diffusion tensor imaging in spinal cord injury. Biomed Sci Instrum. 2008; 44:28–33.24. Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009; 29:2805–2813.25. Maus TP. Imaging of spinal stenosis: neurogenic intermittent claudication and cervical spondylotic myelopathy. Radiol Clin North Am. 2012; 50:651–679.26. Ramanauskas WL, Wilner HI, Metes JJ, Lazo A, Kelly JK. MR imaging of compressive myelomalacia. J Comput Assist Tomogr. 1989; 13:399–404.27. Cook C, Brown C, Isaacs R, Roman M, Davis S, Richardson W. Clustered clinical findings for diagnosis of cervical spine myelopathy. J Man Manip Ther. 2010; 18:175–180.28. Jeong EK, Kim SE, Guo J, Kholmovski EG, Parker DL. High-resolution DTI with 2D interleaved multislice reduced FOV single-shot diffusion-weighted EPI (2D ss-rFOV-DWEPI). Magn Reson Med. 2005; 54:1575–1579.29. Kang Y, Lee JW, Koh YH, Hur S, Kim SJ, Chai JW, et al. New MRI grading system for the cervical canal stenosis. AJR Am J Roentgenol. 2011; 197:W134–W140.30. Jones J, Lerner A, Kim PE, Law M, Hsieh PC. Diffusion tensor imaging in the assessment of ossification of the posterior longitudinal ligament: a report on preliminary results in 3 cases and review of the literature. Neurosurg Focus. 2011; 30:E14.31. Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001; 13:534–546.32. Nevo U, Hauben E, Yoles E, Agranov E, Akselrod S, Schwartz M, et al. Diffusion anisotropy MRI for quantitative assessment of recovery in injured rat spinal cord. Magn Reson Med. 2001; 45:1–9.33. Uda T, Takami T, Sakamoto S, Tsuyuguchi N, Yamagata T, Ohata K. Normal variation of diffusion tensor parameters of the spinal cord in healthy subjects at 3.0-Tesla. J Craniovertebr Junction Spine. 2011; 2:77–78.34. Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry. 2000; 69:269–272.35. Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001; 13(6 Pt 1):1174–1185.36. Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Röther J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004; 22:1767–1774.37. Van Hecke W, Leemans A, Sijbers J, Vandervliet E, Van Goethem J, Parizel PM. A tracking-based diffusion tensor imaging segmentation method for the detection of diffusion-related changes of the cervical spinal cord with aging. J Magn Reson Imaging. 2008; 27:978–991.38. Clark CA, Werring DJ. Diffusion tensor imaging in spinal cord: methods and applications - a review. NMR Biomed. 2002; 15:578–586.39. Ducreux D, Fillard P, Facon D, Ozanne A, Lepeintre JF, Renoux J, et al. Diffusion tensor magnetic resonance imaging and fiber tracking in spinal cord lesions: current and future indications. Neuroimaging Clin N Am. 2007; 17:137–147.
- Full Text Links
- Actions
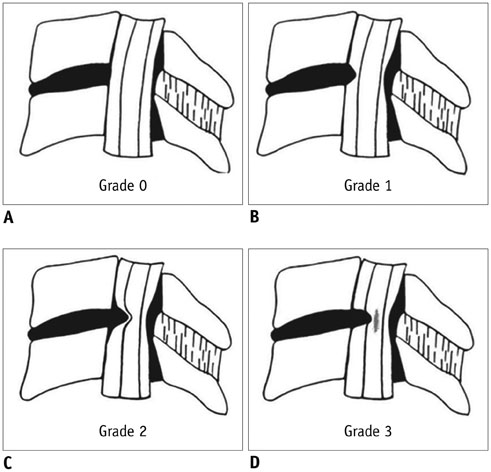
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Metrics as a Potential Prognostic Biomarker in Cervical Myelopathy
- Normal Values of Diffusion Tensor Magnetic Resonance Imaging Parameters in the Cervical Spinal Cord
- The Prediction of Neurological Prognosis for Cervical Spondylotic Myelopathy Using Diffusion Tensor Imaging
- Dynamic Cord Compression Causing Cervical Myelopathy
- The Factors Affecting Surgical Results in Cervical Spondylotic Myelopathy