Korean J Ophthalmol.
2014 Aug;28(4):343-350.
Comparative Quantification of Plasma TDRD7 mRNA in Cataract Patients by Real-time Polymerase Chain Reaction
- Affiliations
-
- 1Department of Ophthalmology, Chosun University College of Medicine, Gwangju, Korea. clearcornea@chosun.ac.kr
- 2Department of Laboratory Medicine, Chosun University College of Medicine, Gwangju, Korea.
Abstract
- PURPOSE
To investigate the relationship between plasma TDRD7 mRNA and lens transparency, and to evaluate plasma TDRD7 mRNA as a potential marker for cataracts and its sub-type by quantitatively analyzing human peripheral blood.
METHODS
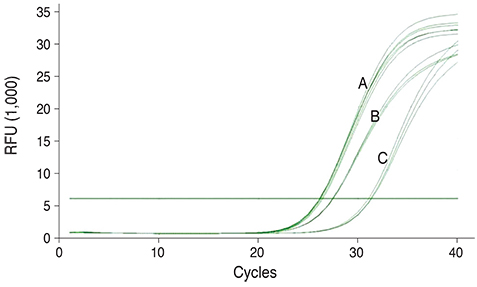
Plasma RNA was extracted from 40 patients with cataracts, and 30 normal controls of matched age and gender. Blood cholesterol and fasting glucose were measured, and the RNA extracted from the sample was synthesized into cDNA. After polymerase chain reaction, the results were compared after quantifying the TDRD7 mRNA using ABL1 mRNA for normalization. We analyzed the relative gene expression data via the DeltaDeltaCt method.
RESULTS
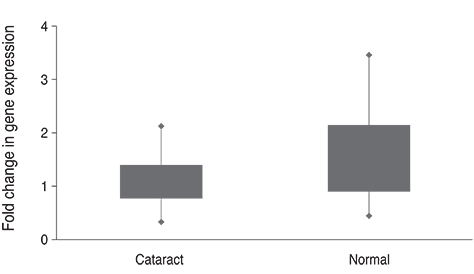
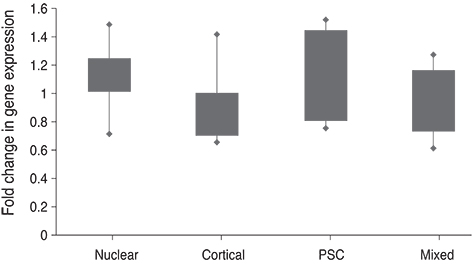
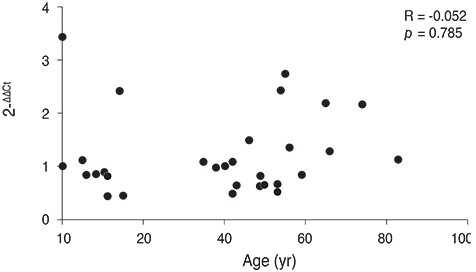
The normalized 2(-DeltaDeltaCt) of plasma TDRD7 mRNA based on ABL1 mRNA was 1.52 ± 0.63 in the case of the control group and 1.05 ± 0.34 in the case of the cataract patients, and the TDRD7 expression level of the cataract patients was lower than that of the control group (p = 0.048). The comparison of the genetic values of different types of cataracts demonstrated that the TDRD7 expression level of the cortical type and mixed type were lower than those of the nuclear type and posterior subcapsular opacity type (p = 0.017).
CONCLUSIONS
Human cataracts and the TDRD7 gene loss-of-function mutations are strongly causally related, as the expression level of plasma TDRD7 mRNA in patients with cataracts was statistically significantly lower than in the normal control group.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Cataract/*blood
Child
Female
Gene Expression Regulation/*physiology
Humans
Male
Middle Aged
Proto-Oncogene Proteins c-abl/genetics
RNA, Messenger/*blood
Real-Time Polymerase Chain Reaction
Ribonucleoproteins/*genetics
Proto-Oncogene Proteins c-abl
RNA, Messenger
Ribonucleoproteins
Figure
Reference
-
1. Yoon KC, Mun GH, Kim SD, et al. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008-2009. Korean J Ophthalmol. 2011; 25:421–433.2. Shyn KH, Kim JC, Kim WS, et al. An epidemiological study of the risk factors contributing to the senile cataractogenesis by the Korean Cooperative Cataract Epidemiology Study Group (KCCESG). J Korean Ophthalmol Soc. 1992; 33:127–134.3. Hodge WG, Whitcher JP, Satariano W. Risk factors for age-related cataracts. Epidemiol Rev. 1995; 17:336–346.4. Ederer F, Hiller R, Taylor HR. Senile lens changes and diabetes in two population studies. Am J Ophthalmol. 1981; 91:381–395.5. Delcourt C, Cristol JP, Tessier F, et al. Risk factors for cortical, nuclear, and posterior subcapsular cataracts: the POLA study. Pathologies Oculaires Liees a l'Age. Am J Epidemiol. 2000; 151:497–504.6. West SK, Valmadrid CT. Epidemiology of risk factors for age-related cataract. Surv Ophthalmol. 1995; 39:323–334.7. Leske MC, Chylack LT Jr, Wu SY. The lens opacities case-control study. Risk factors for cataract. Arch Ophthalmol. 1991; 109:244–251.8. Zetterberg M, Tasa G, Prince JA, et al. Methylenetetrahydrofolate reductase genetic polymorphisms in patients with cataract. Am J Ophthalmol. 2005; 140:932–934.9. Utheim OA, Ritland JS, Utheim TP, et al. Apolipoprotein E genotype and risk for development of cataract and age-related macular degeneration. Acta Ophthalmol. 2008; 86:401–403.10. Zetterberg M, Zetterberg H, Palmer M, et al. Apolipoprotein E polymorphism in patients with cataract. Br J Ophthalmol. 2004; 88:716–718.11. Lachke SA, Alkuraya FS, Kneeland SC, et al. Mutation in the RNA granule component TDRD7 cause cataract and glaucoma. Science. 2011; 331:1571–1576.12. Chylack LT Jr, Wolfe JK, Singer DM, et al. The Longitudinal Study of Cataract Study Group. The lens opacities classification system III. Arch Ophthalmol. 1993; 111:831–836.13. Steinberg EP, Javitt JC, Sharkey PD, et al. The content and cost of cataract surgery. Arch Ophthalmol. 1993; 111:1041–1049.14. Shiels A, Hejtmancik JF. Genetic origins of cataract. Arch Ophthalmol. 2007; 125:165–173.15. Song S, Landsbury A, Dahm R, et al. Functions of the intermediate filament cytoskeleton in the eye lens. J Clin Invest. 2009; 119:1837–1848.16. Donaldson P, Kistler J, Mathias RT. Molecular solutions to mammalian lens transparency. News Physiol Sci. 2001; 16:118–123.17. Wistow G, Bernstein SL, Wyatt MK, et al. Expressed sequence tag analysis of adult human lens for the NEIBank Project: over 2000 non-redundant transcripts, novel genes and splice variants. Mol Vis. 2002; 8:171–184.18. Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009; 10:430–436.19. Fagerholm PP, Philipson BT, Lindstrom B. Normal human lens: the distribution of protein. Exp Eye Res. 1981; 33:615–620.20. Wang X, Garcia CM, Shui YB, Beebe DC. Expression and regulation of alpha-, beta-, and gamma-crystallins in mammalian lens epithelial cells. Invest Ophthalmol Vis Sci. 2004; 45:3608–3619.21. Cavallini GM, Masini C, Chiesi C, et al. Cataract development in a young patient with lathosterolosis: a clinicopathologic case report. Eur J Ophthalmol. 2009; 19:139–142.22. Klein BE, Klein R, Lee KE, Grady LM. Statin use and incident nuclear cataract. JAMA. 2006; 295:2752–2758.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison between Real-Time PCR and Agarose Gel Electrophoresis for DNA Quantification
- Influence of Standard Curves on Relative Quantification using Real-time PCR
- Monitoring of bcr-abl Fusion Transcript Levels by Quantitative Real-Time Polymerase Chain Reaction in Chronic Myeloid Leukemia after Bone Marrow Transplantation
- Real-time Polymerase Chain Reaction (PCR) in Microbiology
- Real-Time Polymerase Chain Reaction Assay for the Diagnosis of Pulmonary Tuberculosis