Korean J Ophthalmol.
2014 Aug;28(4):337-342.
Lateral Geniculate Body Evoked Potentials Elicited by Visual and Electrical Stimulation
- Affiliations
-
- 1Department of Ophthalmology, Wonkwang University Hospital, Wonkwang University School of Medicine, Iksan, Korea. ysyang@wku.ac.kr
- 2Hanson Institute, University of Adelaide, Adelaide, Australia.
Abstract
- PURPOSE
Blind individuals who have photoreceptor loss are known to perceive phosphenes with electrical stimulation of their remaining retinal ganglion cells. We proposed that implantable lateral geniculate body (LGB) stimulus electrode arrays could be used to generate phosphene vision. We attempted to refine the basic reference of the electrical evoked potentials (EEPs) elicited by microelectrical stimulations of the optic nerve, optic tract and LGB of a domestic pig, and then compared it to visual evoked potentials (VEPs) elicited by short-flash stimuli.
METHODS
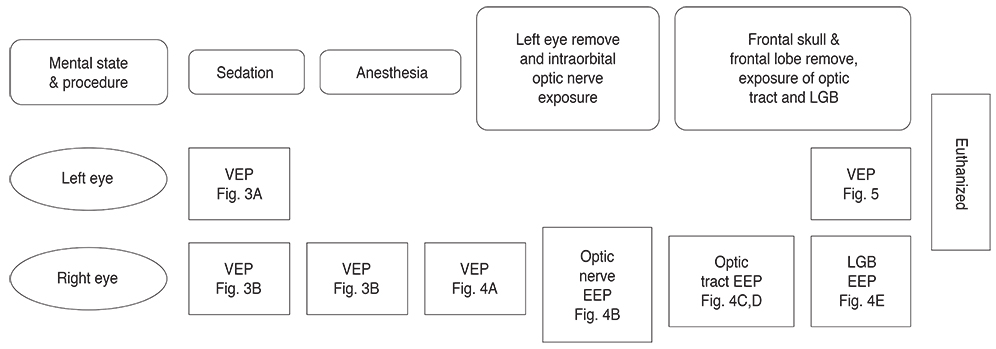
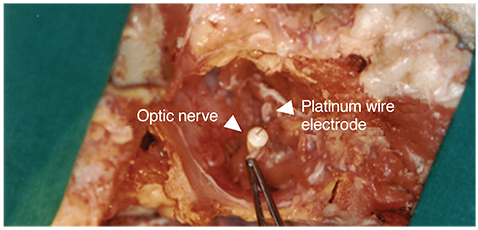
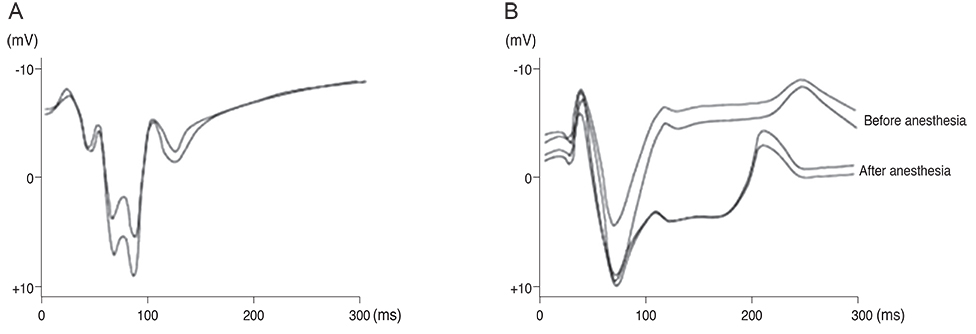
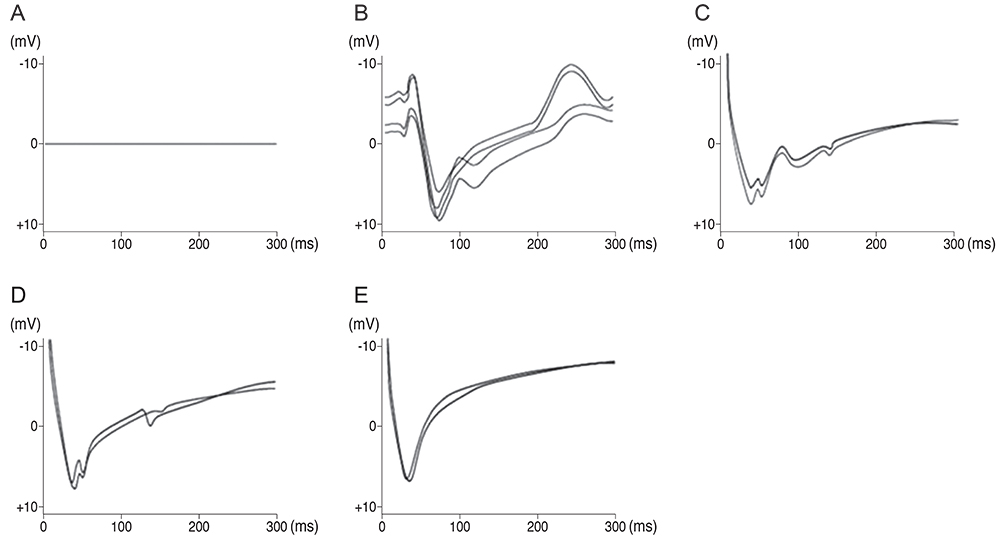
For visual function measurement, VEPs in response to short-flash stimuli on the left eye of the domestic pig were assessed over the visual cortex at position Oz with the reference electrode at Fz. After anesthesia, linearly configured platinum wire electrodes were inserted into the optic nerve, optic track and LGB. To determine the optimal stimulus current, EEPs were recorded repeatedly with controlling the pulse and power. The threshold of current and charge density to elicit EEPs at 0.3 ms pulse duration was about ±10 microA.
RESULTS
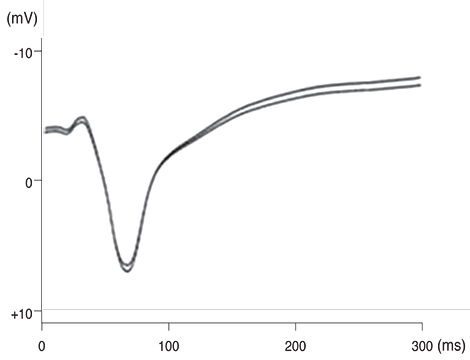
Our experimental results showed that visual cortex activity can be effectively evoked by stimulation of the optic nerve, optic tract and LGB using penetrating electrodes. The latency of P1 was more shortened as the electrical stimulation was closer to LGB. The EEPs of two-channel in the visual cortex demonstrated a similar pattern with stimulation of different spots of the stimulating electrodes. We found that the LGB-stimulated EEP pattern was very similar to the simultaneously generated VEP on the control side, although implicit time deferred.
CONCLUSIONS
EEPs and VEPs derived from visual-system stimulation were compared. The LGB-stimulated EEP wave demonstrated a similar pattern to the VEP waveform except implicit time, indicating prosthetic-based electrical stimulation of the LGB could be utilized for the blind to perceive vision of phosphenes.
MeSH Terms
Figure
Reference
-
1. MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006; 444:203–207.2. Foerster O. Contributions to the pathophysiology of the visual pathway and visual area. J Psychol Neurol. 1929; 39:463–485.3. Krieg WJ. Functional neuroanatomy. 2nd ed. New York: Blakiston;1953. p. 207–208.4. Rizzo JF 3rd, Wyatt J, Loewenstein J, et al. Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short-term surgical trials. Invest Ophthalmol Vis Sci. 2003; 44:5362–5369.5. Zrenner E. Will retinal implants restore vision? Science. 2002; 295:1022–1025.6. Qu J, Wang D, Grosskreutz CL. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res. 2010; 91:48–53.7. Schiefer U, Hart W. Functional anatomy of the human visual pathway. In : Schiefer U, Hart W, Wilhelm H, editors. Clinical neuro-ophthalmology: a practical guide. Berlin: Springer;2007. p. 19–28.8. Krause M, Fogel W, Heck A, et al. Deep brain stimulation for the treatment of Parkinson's disease: subthalamic nucleus versus globus pallidus internus. J Neurol Neurosurg Psychiatry. 2001; 70:464–470.9. Gloesmann M, Hermann B, Schubert C, et al. Histologic correlation of pig retina radial stratification with ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2003; 44:1696–1703.10. Kara P, Pezaris JS, Yurgenson S, Reid RC. The spatial receptive field of thalamic inputs to single cortical simple cells revealed by the interaction of visual and electrical stimulation. Proc Natl Acad Sci U S A. 2002; 99:16261–16266.11. Pezaris JS, Reid RC. Demonstration of artificial visual percepts generated through thalamic microstimulation. Proc Natl Acad Sci U S A. 2007; 104:7670–7675.12. Pezaris JS, Reid RC. Simulations of electrode placement for a thalamic visual prosthesis. IEEE Trans Biomed Eng. 2009; 56:172–178.13. Cohen ED. Prosthetic interfaces with the visual system: biological issues. J Neural Eng. 2007; 4:R14–R31.14. Logothetis NK, Pauls J, Augath M, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001; 412:150–157.15. Schneider KA, Richter MC, Kastner S. Retinotopic organization and functional subdivisions of the human lateral geniculate nucleus: a high-resolution functional magnetic resonance imaging study. J Neurosci. 2004; 24:8975–8985.16. Okada Y, Lahteenmaki A, Xu C. Comparison of MEG and EEG on the basis of somatic evoked responses elicited by stimulation of the snout in the juvenile swine. Clin Neurophysiol. 1999; 110:214–229.17. Laube T, Schanze T, Brockmann C, et al. Chronically implanted epidural electrodes in Gottinger minipigs allow function tests of epiretinal implants. Graefes Arch Clin Exp Ophthalmol. 2003; 241:1013–1019.18. Fang M, Li J, Rudd JA, et al. fMRI mapping of cortical centers following visual stimulation in postnatal pigs of different ages. Life Sci. 2006; 78:1197–1201.19. Gizewski ER, Schanze T, Bolle I, et al. Visualization of the visual cortex in minipigs using fMRI. Res Vet Sci. 2007; 82:281–286.20. Jelsing J, Hay-Schmidt A, Dyrby T, et al. The prefrontal cortex in the Gottingen minipig brain defined by neural projection criteria and cytoarchitecture. Brain Res Bull. 2006; 70:322–336.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Intravenous Anesthetics on Visual Evoked Potentials in Cats
- Amplitude and Latency Difference Between Right and Left Half-Field Visual Evoked Potentials in Normal Subjects
- Intraoperative monitoring of flash visual evoked potential under general anesthesia
- Inluences of transcutaneous electrical nerve stimulation on somatosensory evoked potentials
- The Evoked Potentials Response to Percutaneous Electrical Stimulation in Epilepsy