Cancer Res Treat.
2016 Jul;48(3):1130-1140. 10.4143/crt.2015.206.
Isotype-Specific Inhibition of Histone Deacetylases: Identification of Optimal Targets for Radiosensitization
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea. inah228@snu.ac.kr
- 2Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul, Korea.
- 3Medical Science Research Institute, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2344087
- DOI: http://doi.org/10.4143/crt.2015.206
Abstract
- PURPOSE
Histone deacetylase (HDAC) inhibitors radiosensitize tumor cells. To elucidate mechanisms underlying radiosensitization by HDAC inhibition, understanding of differential contributions of HDAC isotypes is needed. The aim of this study was to investigate involvement of known HDAC isotypes in modulation of cellular radiosensitivity.
MATERIALS AND METHODS
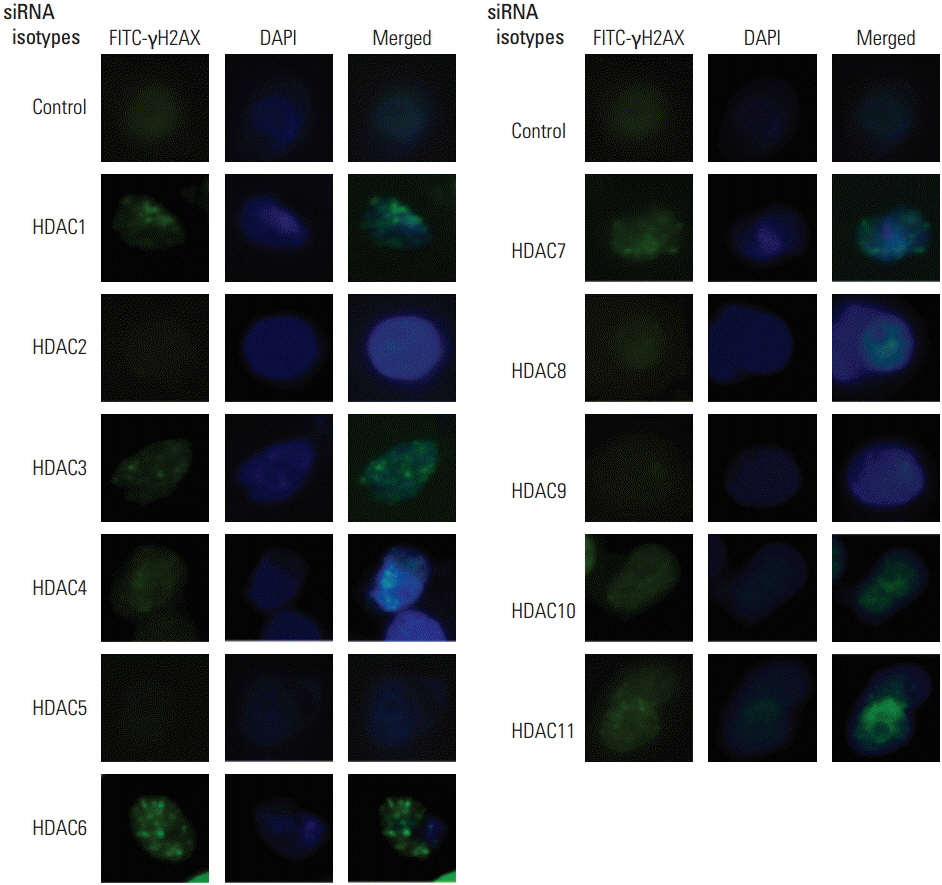
Because pharmacologic HDAC inhibitors lack isotype-specificity, RNA interference against 11 HDAC isotypes was used to inhibit HDAC in an isotype-specific manner. Radiation cell survival was evaluated using a clonogenic assay in SQ20B cells transfected with small interfering RNA specifically targeting HDAC isotypes. Immunocytochemistry was performed for detection of γH2AX foci. Protein expression was measured using Western blotting.
RESULTS
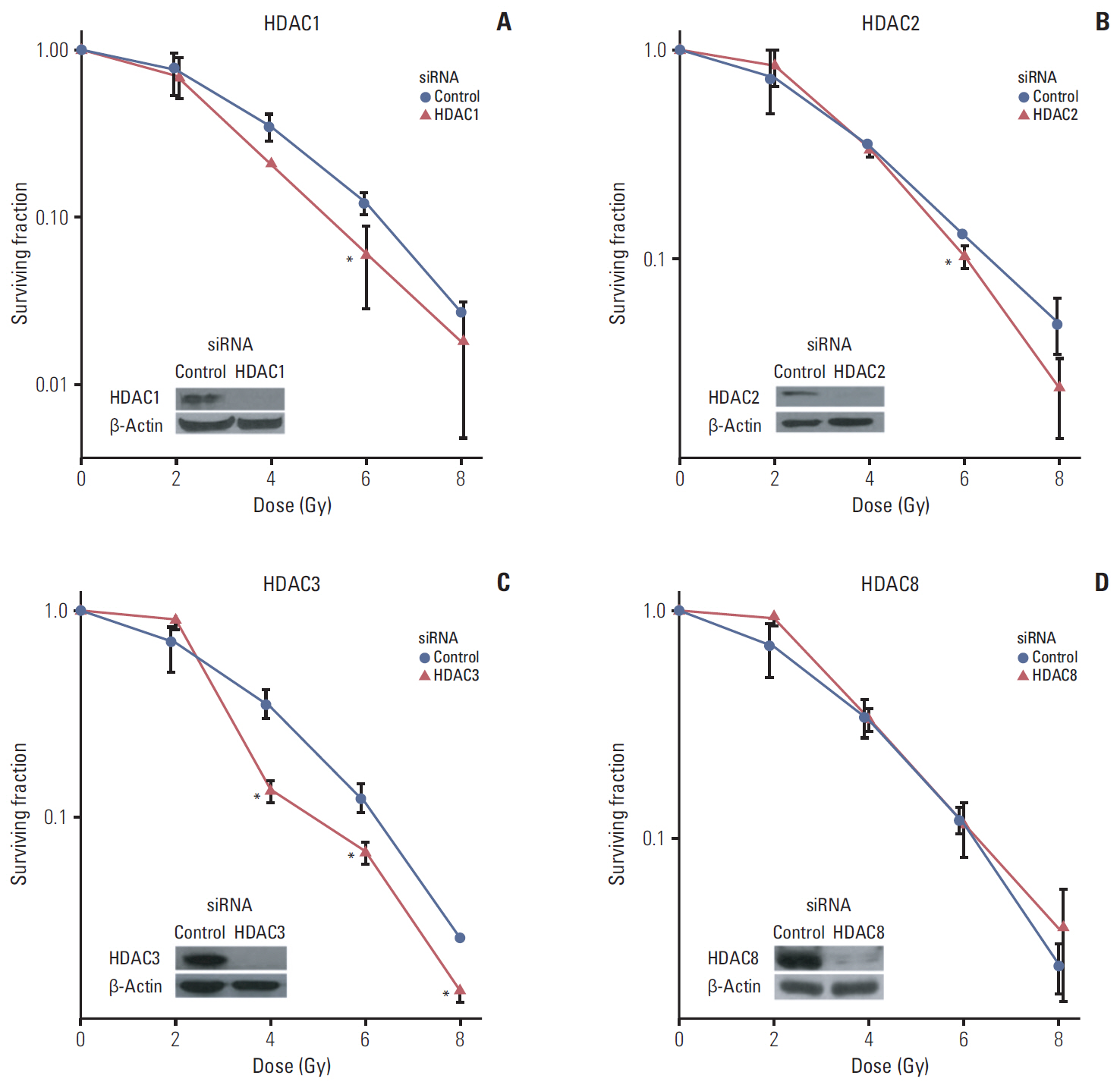
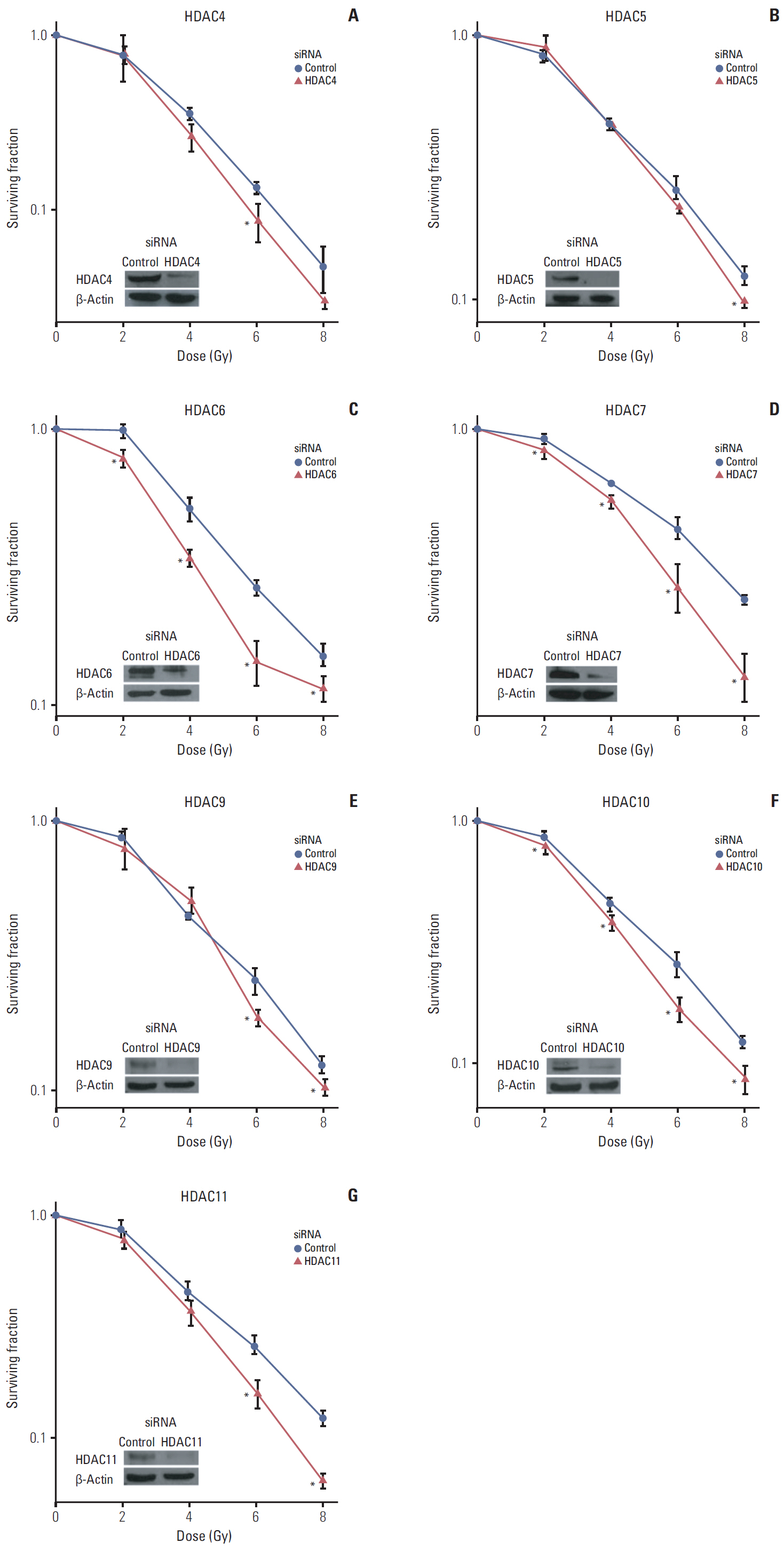
Among 11 HDAC isotypes tested, specific inhibition of 7 isotypes (HDAC1, HDAC3, HDAC4, HDAC6, HDAC7, HDAC10, and HDAC11) enhanced radiation lethality in SQ20B cells. Radiosensitization by inhibition of these HDAC isotypes was accompanied by delay of DNA double strand break repair. Radiosensitivity of SQ20B cells was not altered by selective inhibition of the remaining four isotypes (HDAC2, HDAC5, HDAC8, and HDAC9). Inhibition of HDAC isotypes resulted in downregulation of various proteins involved in pro-survival and DNA damage repair pathways.
CONCLUSION
Isotype-specificity exists in HDAC inhibition-induced radiosensitization. Different HDAC isotypes are differentially involved in modulation of cellular radiosensitivity.
MeSH Terms
Figure
Cited by 2 articles
-
Disulfiram, a Re-positioned Aldehyde Dehydrogenase Inhibitor, Enhances Radiosensitivity of Human Glioblastoma Cells
In Vitro
Hyeon Kang Koh, Soo Yeon Seo, Jin Ho Kim, Hak Jae Kim, Eui Kyu Chie, Seung-Ki Kim, Il Han Kim
Cancer Res Treat. 2019;51(2):696-705. doi: 10.4143/crt.2018.249.Synergistic Tumoricidal Effects of Alpha-Lipoic Acid and Radiotherapy on Human Breast Cancer Cells via HMGB1
Hoon Sik Choi, Jin Hyun Kim, Si Jung Jang, Jeong Won Yun, Ki Mun Kang, Hojin Jeong, In Bong Ha, Bae Kwon Jeong
Cancer Res Treat. 2021;53(3):685-694. doi: 10.4143/crt.2020.1015.
Reference
-
References
1. Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: what are the cancer relevant targets? Cancer Lett. 2009; 277:8–21.
Article2. Lopez CA, Feng FY, Herman JM, Nyati MK, Lawrence TS, Ljungman M. Phenylbutyrate sensitizes human glioblastoma cells lacking wild-type p53 function to ionizing radiation. Int J Radiat Oncol Biol Phys. 2007; 69:214–20.
Article3. Banuelos CA, Banath JP, MacPhail SH, Zhao J, Reitsema T, Olive PL. Radiosensitization by the histone deacetylase inhibitor PCI-24781. Clin Cancer Res. 2007; 13(22 Pt 1):6816–26.
Article4. Geng L, Cuneo KC, Fu A, Tu T, Atadja PW, Hallahan DE. Histone deacetylase (HDAC) inhibitor LBH589 increases duration of gamma-H2AX foci and confines HDAC4 to the cytoplasm in irradiated non-small cell lung cancer. Cancer Res. 2006; 66:11298–304.5. Munshi A, Tanaka T, Hobbs ML, Tucker SL, Richon VM, Meyn RE. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-H2AX foci. Mol Cancer Ther. 2006; 5:1967–74.6. Munshi A, Kurland JF, Nishikawa T, Tanaka T, Hobbs ML, Tucker SL, et al. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res. 2005; 11:4912–22.
Article7. Camphausen K, Cerna D, Scott T, Sproull M, Burgan WE, Cerra MA, et al. Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer. 2005; 114:380–6.8. Kim JH, Shin JH, Kim IH. Susceptibility and radiosensitization of human glioblastoma cells to trichostatin A, a histone deacetylase inhibitor. Int J Radiat Oncol Biol Phys. 2004; 59:1174–80.
Article9. Camphausen K, Burgan W, Cerra M, Oswald KA, Trepel JB, Lee MJ, et al. Enhanced radiation-induced cell killing and prolongation of gammaH2AX foci expression by the histone deacetylase inhibitor MS-275. Cancer Res. 2004; 64:316–21.10. Camphausen K, Scott T, Sproull M, Tofilon PJ. Enhancement of xenograft tumor radiosensitivity by the histone deacetylase inhibitor MS-275 and correlation with histone hyperacetylation. Clin Cancer Res. 2004; 10(18 Pt 1):6066–71.
Article11. Kim IA, Shin JH, Kim IH, Kim JH, Kim JS, Wu HG, et al. Histone deacetylase inhibitor-mediated radiosensitization of human cancer cells: class differences and the potential influence of p53. Clin Cancer Res. 2006; 12(3 Pt 1):940–9.
Article12. Kim IA, Bae SS, Fernandes A, Wu J, Muschel RJ, McKenna WG, et al. Selective inhibition of Ras, phosphoinositide 3 kinase, and Akt isoforms increases the radiosensitivity of human carcinoma cell lines. Cancer Res. 2005; 65:7902–10.
Article13. Cha TL, Chuang MJ, Wu ST, Sun GH, Chang SY, Yu DS, et al. Dual degradation of aurora A and B kinases by the histone deacetylase inhibitor LBH589 induces G2-M arrest and apoptosis of renal cancer cells. Clin Cancer Res. 2009; 15:840–50.
Article14. Hehlgans S, Petraki C, Reichert S, Cordes N, Rodel C, Rodel F. Double targeting of Survivin and XIAP radiosensitizes 3D grown human colorectal tumor cells and decreases migration. Radiother Oncol. 2013; 108:32–9.
Article15. Kao GD, McKenna WG, Guenther MG, Muschel RJ, Lazar MA, Yen TJ. Histone deacetylase 4 interacts with 53BP1 to mediate the DNA damage response. J Cell Biol. 2003; 160:1017–27.
Article16. Kim JH, Kim IH, Shin JH, Kim HJ, Kim IA. Sequence-dependent radiosensitization of histone deacetylase inhibitors trichostatin A and SK-7041. Cancer Res Treat. 2013; 45:334–42.
Article17. Litvinov SV. Main repair pathways of double-strand breaks in the genomic DNA and interactions between them. Cytol Genet. 2014; 48:189–202.
Article18. Kim GD, Choi YH, Dimtchev A, Jeong SJ, Dritschilo A, Jung M. Sensing of ionizing radiation-induced DNA damage by ATM through interaction with histone deacetylase. J Biol Chem. 1999; 274:31127–30.
Article19. Keen N, Taylor S. Aurora-kinase inhibitors as anticancer agents. Nat Rev Cancer. 2004; 4:927–36.
Article20. Lin ZZ, Chou CH, Cheng AL, Liu WL, Chia-Hsien Cheng J. Radiosensitization by combining an aurora kinase inhibitor with radiotherapy in hepatocellular carcinoma through cell cycle interruption. Int J Cancer. 2014; 135:492–501.
Article21. Tao Y, Zhang P, Girdler F, Frascogna V, Castedo M, Bourhis J, et al. Enhancement of radiation response in p53-deficient cancer cells by the Aurora-B kinase inhibitor AZD1152. Oncogene. 2008; 27:3244–55.
Article22. Rodel F, Hoffmann J, Distel L, Herrmann M, Noisternig T, Papadopoulos T, et al. Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res. 2005; 65:4881–7.
Article23. Moussata D, Amara S, Siddeek B, Decaussin M, Hehlgans S, Paul-Bellon R, et al. XIAP as a radioresistance factor and prognostic marker for radiotherapy in human rectal adenocarcinoma. Am J Pathol. 2012; 181:1271–8.
Article24. Cao C, Mu Y, Hallahan DE, Lu B. XIAP and survivin as therapeutic targets for radiation sensitization in preclinical models of lung cancer. Oncogene. 2004; 23:7047–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epigenetic Modifications: Novel Therapeutic Approach for Thyroid Cancer
- Sequence-Dependent Radiosensitization of Histone Deacetylase Inhibitors Trichostatin A and SK-7041
- Dual Inhibitors Against Topoisomerases and Histone Deacetylases
- HDAC and HDAC Inhibitor: From Cancer to Cardiovascular Diseases
- Histone Deacetylases and Their Regulatory MicroRNAs in Hepatocarcinogenesis