Cancer Res Treat.
2016 Jul;48(3):1120-1129. 10.4143/crt.2015.436.
A Multi-centric Bioequivalence Trial in Ph+ Chronic Myeloid Leukemia Patients to Assess Bioequivalence and Safety Evaluation of Generic Imatinib Mesylate 400 mg Tablets
- Affiliations
-
- 1Faculty of Pharmacy, Jamia Hamdard, Hamdard University, New Delhi, India. rachna.arora@sunpharma.com
- 2Clinical Pharmacology and Pharmacokinetics, Sun Pharmaceutical Industries Limited, Gurgaon, Haryana, India.
- KMID: 2344086
- DOI: http://doi.org/10.4143/crt.2015.436
Abstract
- PURPOSE
This study was designed to characterize the pharmacokinetic profile and to assess bioequivalence of the sponsor's test formulation (imatinib mesylate 400 mg tablets) with an innovator product (Gleevec 400 mg tablets, Novartis Pharmaceuticals) under fed conditions, in adult patients of Philadelphia chromosome positive chronic myeloid leukemia (Ph+ CML) stabilized on imatinib mesylate 400 mg. In addition, the aim of this study was to monitor the safety profile of investigational medicinal products (IMPs).
MATERIALS AND METHODS
A multicenter, randomized, open label, two-period, crossover, single dose bioequivalence study was designed for conduct under fed conditions in 42 adult Ph+ CML patients already stabilized on imatinib 400 mg tablets. Pharmacokinetic parameters Tmax, Cmax, and AUC0-24 were calculated using a non-compartmental model on validated WinNonlin software. Validated SAS software was used for statistical evaluation of data. The safety profile of investigational products was monitored during the course of study by applying a clinical process for recording observed untoward effects postadministration of investigational products.
RESULTS
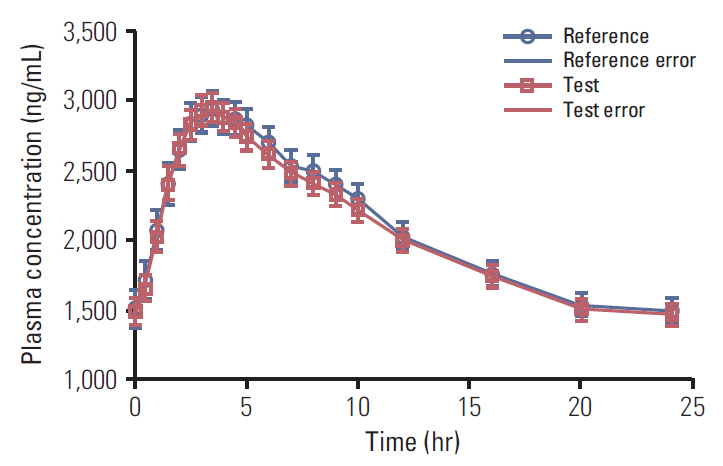
The 90% confidence intervals for the test/reference mean ratios of the ln-transformed PK variables Cmax (99.0%) and AUC0-24 (99.2%) were within an acceptable range of 80%-125%, as per bioequivalence assumptions. Both formulations were well tolerated after oral administration of IMPs.
CONCLUSION
The test product was found to be bioequivalent and safe, and thus can be used interchangeably in clinical practice.
MeSH Terms
Figure
Cited by 1 articles
-
Use of generic imatinib as first-line treatment in patients with chronic myeloid leukemia (CML): the GIMS (Glivec to Imatinib Switch) study
Maria Gemelli, Elena Maria Elli, Chiara Elena, Alessandra Iurlo, Tamara Intermesoli, Margherita Maffioli, Ester Pungolino, Maria Cristina Carraro, Mariella D’Adda, Francesca Lunghi, Michela Anghileri, Nicola Polverelli, Marianna Rossi, Mattia Bacciocchi, Elisa Bono, Cristina Bucelli, Francesco Passamonti, Laura Antolini, Carlo Gambacorti-Passerini
Blood Res. 2020;55(3):139-145. doi: 10.5045/br.2020.2020130.
Reference
-
References
1. Robertson SC, Tynan JA, Donoghue DJ. RTK mutations and human syndromes: when good receptors turn bad. Trends Genet. 2000; 16:265–71.
Article2. Gora-Tybor J, Robak T. Targeted drugs in chronic myeloid leukemia. Curr Med Chem. 2008; 15:3036–51.
Article3. Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008; 112:4808–17.
Article4. Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999; 341:164–72.
Article5. O'Dwyer ME, Druker BJ. Chronic myelogenous leukaemia: new therapeutic principles. J Intern Med. 2001; 250:3–9.6. Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999; 340:1330–40.
Article7. Guilhot F, Chastang C, Michallet M, Guerci A, Harousseau JL, Maloisel F, et al. Interferon alfa-2b combined with cytarabine versus interferon alone in chronic myelogenous leukemia. French Chronic Myeloid Leukemia Study Group. N Engl J Med. 1997; 337:223–9.8. Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000; 295:139–45.9. Borg C, Terme M, Taieb J, Menard C, Flament C, Robert C, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004; 114:379–88.
Article10. Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003; 21:4342–9.
Article11. Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001; 344:1031–7.
Article12. Nikolova Z, Peng B, Hubert M, Sieberling M, Keller U, Ho YY, et al. Bioequivalence, safety, and tolerability of imatinib tablets compared with capsules. Cancer Chemother Pharmacol. 2004; 53:433–8.
Article13. Peng B, Dutreix C, Mehring G, Hayes MJ, Ben-Am M, Seiberling M, et al. Absolute bioavailability of imatinib (Glivec) orally versus intravenous infusion. J Clin Pharmacol. 2004; 44:158–62.
Article14. Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005; 44:879–94.
Article15. Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001; 344:1038–42.
Article16. van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009; 35:692–706.
Article17. Gschwind HP, Pfaar U, Waldmeier F, Zollinger M, Sayer C, Zbinden P, et al. Metabolism and disposition of imatinib mesylate in healthy volunteers. Drug Metab Dispos. 2005; 33:1503–12.
Article18. Ault P, Kantarjian H, O'Brien S, Faderl S, Beran M, Rios MB, et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2006; 24:1204–8.
Article19. O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003; 348:994–1004.20. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472–80.
Article21. Smith AG, Painter D, Howell DA, Evans P, Smith G, Patmore R, et al. Determinants of survival in patients with chronic myeloid leukaemia treated in the new era of oral therapy: findings from a UK population-based patient cohort. BMJ Open. 2014; 4:e004266.
Article22. Drummond M. Pharmacoeconomics: friend or foe? Ann Rheum Dis. 2006; 65 Suppl 3:iii44–7.
Article23. Center for Drug Evaluation and Research. Guidance for industry: bioanalytical method validation. Silver Spring, MD: U. S. Department of Health and Human Services, Food and Drug Administration;2001.24. Light DW, Lexchin J. Why do cancer drugs get such an easy ride? BMJ. 2015; 350:h2068.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Complete remission of philadelphia chromosome-positive acute myeloid leukemia with imatinib mesylate
- Fed and fasted bioequivalence assessment of two formulations of extended-release fixed-dose combination dapagliflozin/metformin (10/1,000 mg) tablets in healthy subjects
- Establishment and Significance of Bioequivalence Recommendations for Individual Products-Drugs Acting on Circulatory System and Others
- Peripheral neuropathy associated with imatinib therapy for chronic myeloid leukemia
- Three cases of Philadelphia (Ph) chromosome positive acute myeloid leukemia (AML) treated with imatinib mesylate (Glivec, STI571) and allogeneic hematopoietic stem cell transplantation after induction chemotherapy