Cancer Res Treat.
2016 Jul;48(3):917-927. 10.4143/crt.2015.265.
The Role of Neoadjuvant Chemotherapy in the Treatment of Nasopharyngeal Carcinoma: A Multi-institutional Retrospective Study (KROG 11-06) Using Propensity Score Matching Analysis
- Affiliations
-
- 1Department of Radiation Oncology, Gyeongsang National University Hospital and Gyeongsang National University School of Medicine, Jinju, Korea.
- 2Department of Radiation Oncology, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Otolaryngology-Head and Neck Surgery, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Radiation Oncology, Yonsei University College of Medicine, Seoul, Korea.
- 7Department of Radiation Oncology, Research Institute and Hospital, National Cancer Center, Seoul, Korea.
- 8Department of Radiation Oncology, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 9Department of Radiation Oncology, Chonnam National University Medical School, Gwangju, Korea.
- 10Department of Radiation Oncology, Ajou University School of Medicine, Suwon, Korea.
- 11Department of Radiation Oncology, Pusan National University School of Medicine, Busan, Korea.
- 12Department of Radiation Oncology, Chungnam National University School of Medicine, Daejeon, Korea.
- 13Department of Radiation Oncology, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea.
- 14Department of Radiation Oncology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. yeonkim7@catholic.ac.kr
- KMID: 2344064
- DOI: http://doi.org/10.4143/crt.2015.265
Abstract
- PURPOSE
We compared the treatment results and toxicity in nasopharyngeal carcinoma (NPC) patients treated with concurrent chemotherapy (CCRT) alone (the CRT arm) or neoadjuvant chemotherapy followed by CCRT (the NCT arm).
MATERIALS AND METHODS
A multi-institutional retrospective study was conducted to review NPC patterns of care and treatment outcome. Data of 568 NPC patients treated by CCRT alone or by neoadjuvant chemotherapy followed by CCRT were collected from 15 institutions. Patients in both treatment arms were matched using the propensity score matching method, and the clinical outcomes were analyzed.
RESULTS
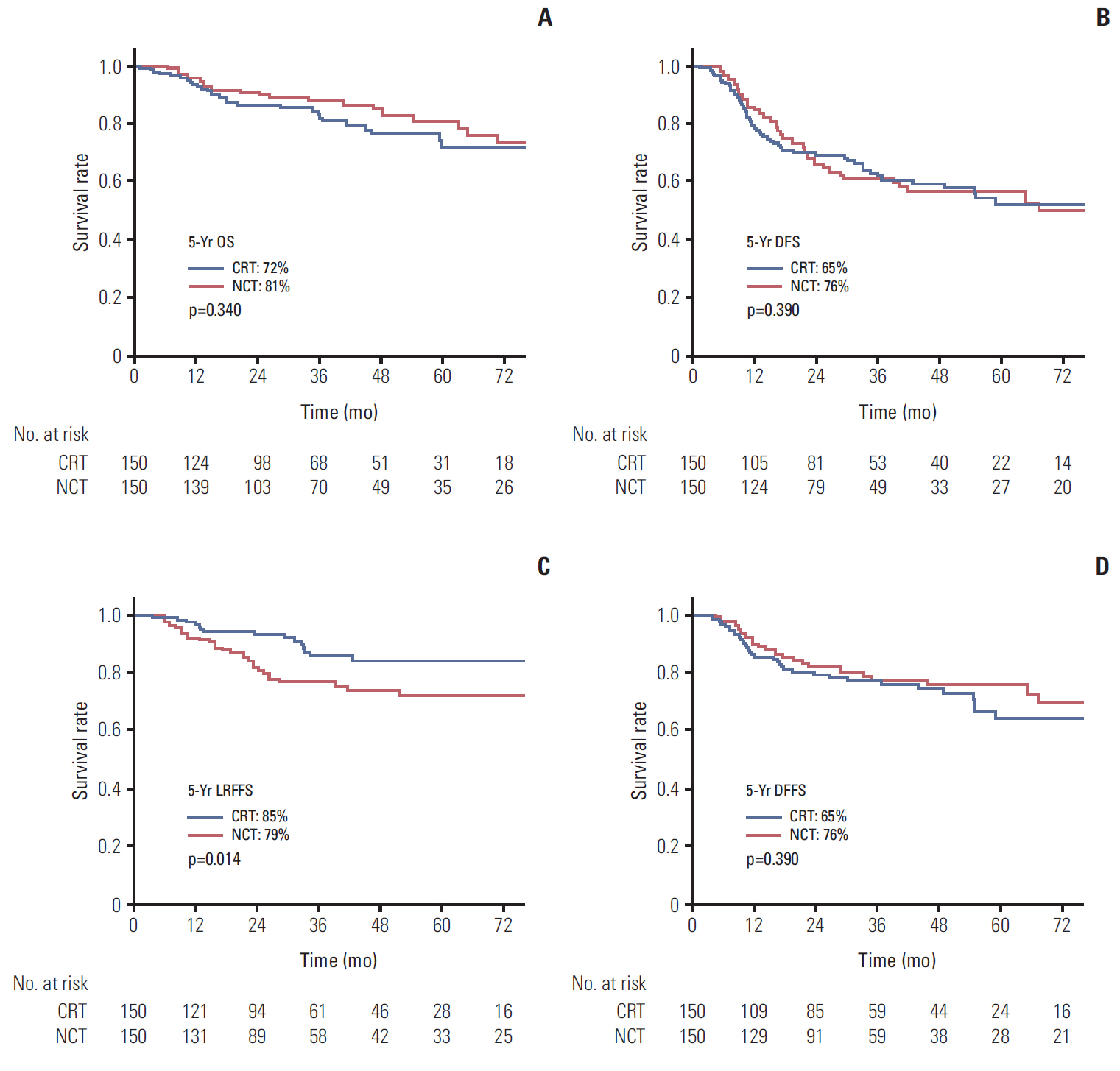
After matching, 300 patients (150 patients in each group) were selected for analysis. Higher 5-year locoregional failure-free survival was observed in the CRT arm (85% vs. 72%, p=0.014). No significant differences in distant failure-free survival (DFFS), disease-free survival (DFS), and overall survival were observed between groups. In subgroup analysis, the NCT arm showed superior DFFS and DFS in stage IV patients younger than 60 years. No significant difference in compliance and toxicity was observed between groups, except the radiation therapy duration was slightly shorter in the CRT arm (50.0 days vs. 53.9 days, p=0.018).
CONCLUSION
This study did not show the superiority of NCT followed by CCRT over CCRT alone. Because NCT could increase the risk of locoregional recurrences, it can only be considered in selected young patients with advanced stage IV disease. The role of NCT remains to be defined and should not be viewed as the standard of care.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Longitudinal Assessment of Intravoxel Incoherent Motion Diffusion Weighted Imaging in Evaluating the Radio-sensitivity of Nasopharyngeal Carcinoma Treated with Intensity-Modulated Radiation Therapy
Youping Xiao, Ying Chen, Yunbin Chen, Zhuangzhen He, Yiqi Yao, Jianji Pan
Cancer Res Treat. 2019;51(1):345-356. doi: 10.4143/crt.2018.089.
Reference
-
References
1. Jeon YK, Lee BY, Kim JE, Lee SS, Kim CW. Molecular characterization of Epstein-Barr virus and oncoprotein expression in nasopharyngeal carcinoma in Korea. Head Neck. 2004; 26:573–83.
Article2. Kim YJ, Go H, Wu HG, Jeon YK, Park SW, Lee SH. Immunohistochemical study identifying prognostic biomolecular markers in nasopharyngeal carcinoma treated by radiotherapy. Head Neck. 2011; 33:1458–66.
Article3. The Korea Central Cancer Registry; National Cancer Center. Annual report of cancer statistics in Korea in 2011. Gwacheon: Ministry of Health and Welfare;2012.4. Lee NK, Park YJ, Yang DS, Yoon WS, Lee S, Kim CY. Long-term results of 2-dimensional radiation therapy in patients with nasopharyngeal cancer. J Korean Soc Ther Radiol Oncol. 2010; 28:193–204.
Article5. Spratt DE, Lee N. Current and emerging treatment options for nasopharyngeal carcinoma. Onco Targets Ther. 2012; 5:297–308.6. Kim YS, Kim BS, Jung SL, Lee YS, Kim MS, Sun DI, et al. Radiation therapy combined with (or without) cisplatin-based chemotherapy for patients with nasopharyngeal cancer: 15-years experience of a single institution in Korea. Cancer Res Treat. 2008; 40:155–63.
Article7. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998; 16:1310–7.
Article8. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003; 21:631–7.
Article9. Chan AT, Leung SF, Ngan RK, Teo PM, Lau WH, Kwan WH, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005; 97:536–9.
Article10. Kong M, Hong SE, Choi J, Kim Y. Comparison of survival rates between patients treated with conventional radiotherapy and helical tomotherapy for head and neck cancer. Radiat Oncol J. 2013; 31:1–11.
Article11. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009; 27:242–9.
Article12. Bae WK, Hwang JE, Shim HJ, Cho SH, Lee JK, Lim SC, et al. Phase II study of docetaxel, cisplatin, and 5-FU induction chemotherapy followed by chemoradiotherapy in locoregionally advanced nasopharyngeal cancer. Cancer Chemother Pharmacol. 2010; 65:589–95.
Article13. Ekenel M, Keskin S, Basaran M, Ozdemir C, Meral R, Altun M, et al. Induction chemotherapy with docetaxel and cisplatin is highly effective for locally advanced nasopharyngeal carcinoma. Oral Oncol. 2011; 47:660–4.
Article14. Fountzilas G, Ciuleanu E, Bobos M, Kalogera-Fountzila A, Eleftheraki AG, Karayannopoulou G, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol. 2012; 23:427–35.
Article15. Liang ZG, Zhu XD, Tan AH, Jiang YM, Qu S, Su F, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy with or with-out adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: meta-analysis of 1,096 patients from 11 randomized controlled trials. Asian Pac J Cancer Prev. 2013; 14:515–21.
Article16. Wee CW, Keam B, Heo DS, Sung MW, Won TB, Wu HG. Locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy plus concurrent weekly cisplatin with or without neoadjuvant chemotherapy. Radiat Oncol J. 2015; 33:98–108.
Article17. Thompson LD. Update on nasopharyngeal carcinoma. Head Neck Pathol. 2007; 1:81–6.
Article18. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47:207–14.
Article19. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003; 13:176–81.
Article20. Kong L, Hu C, Niu X, Zhang Y, Guo Y, Tham IW, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiation for locoregionally advanced nasopharyngeal carcinoma: interim results from 2 prospective phase 2 clinical trials. Cancer. 2013; 119:4111–8.21. Ma J, Mai HQ, Hong MH, Min HQ, Mao ZD, Cui NJ, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001; 19:1350–7.
Article22. Hareyama M, Sakata K, Shirato H, Nishioka T, Nishio M, Suzuki K, et al. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer. 2002; 94:2217–23.
Article23. Zhang LN, Gao YH, Lan XW, Tang J, OuYang PY, Xie FY. Effect of taxanes-based induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: A large scale propensity-matched study. Oral Oncol. 2015; 51:950–6.
Article24. Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007; 357:1695–704.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Re-Excision Rate in Breast Conservation Surgery after Neoadjuvant Chemotherapy
- Applications of propensity score matching: a case series of articles published in Annals of Coloproctology
- Individualized Concurrent Chemotherapy for Patients with Stage III-IVa Nasopharyngeal Carcinoma Receiving Neoadjuvant Chemotherapy Combined with Definitive Intensity-Modulated Radiotherapy
- The Prognostic Value of Albumin-to-Alkaline Phosphatase Ratio before Radical Radiotherapy in Patients with Non-metastatic Nasopharyngeal Carcinoma: A Propensity Score Matching Analysis
- Clinical Outcomes of Neoadjuvant Chemotherapy in Colorectal Cancer Patients With Synchronous Resectable Liver Metastasis: A Propensity Score Matching Analysis