J Korean Ophthalmol Soc.
2014 Aug;55(8):1195-1201.
Expression of TonEBP by Hypertonic and Hyperosmolar Stress in RGC-5 Cells
- Affiliations
-
- 1Department of Ophthalmology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. limbus68@naver.com
- 2School of Biological Sciences, College of Natural Science, University of Ulsan, Ulsan, Korea.
Abstract
- PURPOSE
In order to determine whether the Tonicity responsive enhancer binding protein (TonEBP) is expressed by hypertonic and hyperosmolar stress, TonEBP expression was investigated in the retinal ganglion cell (RGC) line, RGC-5 cells.
METHODS
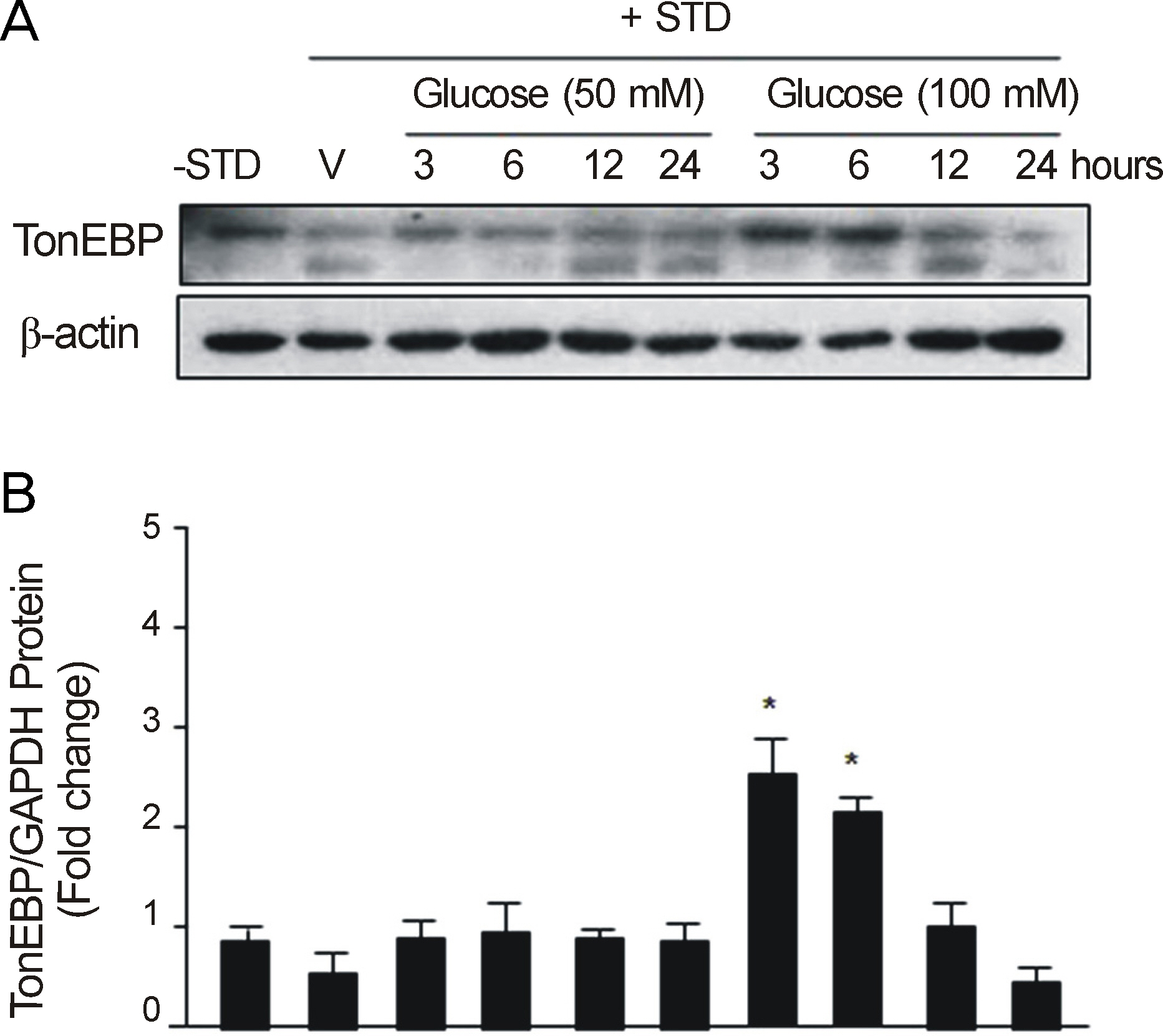
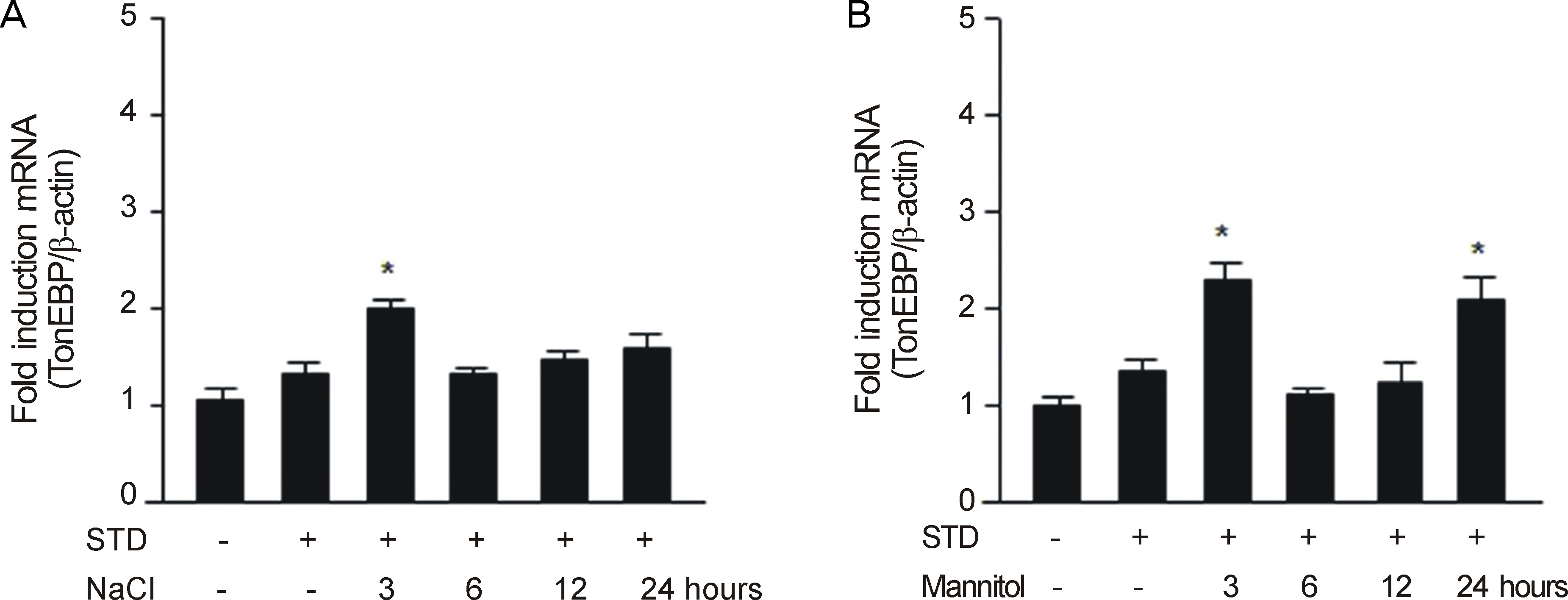
After RGC-5 cells were cultured by Staurosporine, TonEBP expression was measured with Western immunoblotting analysis and real-time reverse transcription-polymerase chain reaction in 50 mM NaCl, 100 mM mannitol, 50 mM glucose, or 100 mM glucose at 3, 6, 12, and 24 hours after exposure to each environment.
RESULTS
In this study, the protein expression of TonEBP was determined to be statistically significantly checked in 50 mM NaCl after 3, and 6 hours, in 100 mM mannitol after 6 hours, and in 100 mM glucose after 3, and 6 hours. TonEBP messenger Ribonucleic acid (mRNA) expression was determined to be statistically significantly checked in 50 mM NaCl after 3 hours, in 100 mM mannitol after 3, and 24 hours, and in 50 mM glucose after 3, and 24 hours.
CONCLUSIONS
These results suggested that TonEBP was expressed by hypertonic and hyperosmolar stress at the protein and mRNA levels. Further studies are nedded to determine the role of TonEBP and the mechanism of expression and regulation of TonEBP.
MeSH Terms
Figure
Reference
-
References
1. The Advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130:429–40.2. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma study group. Am J Ophthalmol. 1998; 126:487–97.3. Chen JZ, Kadlubar FF. A new clue to glaucoma pathogenesis. Am J Med. 2003; 114:697–8.
Article4. Flammer J, Haefliger IO, Orgul S, Resink T. Vascular dysregulation: a principal risk factor for glaucoma damage. J Glaucoma. 1999; 8:212–9.5. Matsumoto S, Shamloo M, Matsumoto E, et al. Protein kinase C-gamma and calcium/calmodulin-dependent protein kinase II-alpha are persistently translocated to cell membranes of the rat brain during and after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2004; 24:54–61.6. Speechly-Dick ME, Mocanu MM, Yellon DM. Protein kinase C. Its role in ischemic preconditioning in the rat. Circ Res. 1994; 75:586–90.
Article7. Piccoletti R, Bendinelli P, Arienti D, Bernelli-Zazzera A. State and activity of protein kinase C in postischemic reperfused liver. Exp Mol Pathol. 1992; 56:219–28.
Article8. Padanilam BJ. Induction and subcellular localization of protein kinase C isozymes following renal ischemia. Kidney Int. 2001; 59:1789–97.
Article9. Tanaka C, Nichizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994; 17:551–67.
Article10. Chen L, Hahn H, Wu G, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA. 2001; 98:11114–9.11. Whitlock NA, Agarwal N, Ma JX, Crosson CE. Hsp27 upregulation by HIF-1 signaling offers protection against retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2005; 46:1092–8.
Article12. McNaughts KS, Carrupt PA, Altomare C, et al. Isoquinoline derivatives as endogenous neurotoxins in the etiology of Parkinson's disease. Biochem Pharmacol. 1998; 56:921–33.13. Cunha-Vaz J, Faria de Abreu JR, Campos AJ. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975; 59:649–56.
Article14. King GL, Brownlee M. The cellular and molecular mechanisms of diabetic complications. Endocrinol Metab Clin North Am. 1996; 25:255–70.
Article15. Barber AJ, Nakamura M, Wolpert EB, et al. Insulin rescues retinal neurons from apoptosis by a phophatidylinositol 3-kinase/Akt- mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 2001; 276:32814–21.16. Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signaling in cell volume regulation. Int Rev Cytol. 1995; 161:173–262.17. Nakayama Y, Peng T, Sands JM, Bagnasco SM. The TonE/TonEBP pathway mediates tonicity-responsive regulation of UT-A urea transporter expression. J Biol Chem. 2000; 275:38275–80.
Article18. Woo SK, Lee SD, Na KY, et al. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol. 2002; 22:5753–60.
Article19. Cha JH, Woo SK, Han KH, et al. Hydration status affects nuclear distribution of transcription factor tonicity responsive enhancer binding protein in rat kidney. J Am Soc Nephrol. 2001; 12:2221–30.
Article20. Lopez-Rodriguez C, Aramburu J, Jin L, et al. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001; 15:47–58.21. Zhang Z, Ferraris JD, Brooks HL, et al. Expression of osmotic stress-related genesin tissues of normal and hypoosmotic rats. Am J Physiol Renal Physiol. 2003; 285:F688–93.22. Favale NO, Casali CI, Lepera LG, et al. Hypertonic induction of COX2 expression requires TonEBP/NFAT5 in renal epithelial cells. Biochem and Biophys Res Commun. 2009; 381:301–5.
Article23. Roth I, Leroy V, Kwon HM, et al. Osmoprotective transcription factor NFAT5/TonEBP modulates nuclear factor-kappaB activity. Mol Biol Cell. 2010; 21:3459–74.24. Woo SK, Lee SD, Na KY, et al. TonEBP/NFAT5 stimulates transcription of HSP70 in Response to Hypertonicity. Mol Biol Cell. 2002; 22:5753–60.
Article25. Lee SD, Choi SY, Lim SW, et al. TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress: organic osmolyte-dependent and -independent pathways. Am J Physiol Renal Physiol. 2011; 300:F707–15.
Article26. Trama J, Lu Q, Hawley RG, Ho SN. The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J Immunol. 2000; 165:4884–94.
Article27. Jauliac S, Lopez-Rodriguez C, Shaw LM, et al. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002; 4:540–4.
Article28. Trama J, Go WY, Ho SN. The osmoprotective function of the NFAT5 transcription factor in T cell development and activation. J Immunol. 2002; 169:5477–88.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Aralia Elata on the Expression of Hyperosmolarity-induced TonEBP Protein and Inflammatory Mediators in Corneal Epithelial Cells
- The Abundance and Nuclear Distribution of TonEBP in Response to Changes of Tonicity in Hyperglycemic Cells
- TonEBP suppresses adipocyte differentiation via modulation of early signaling in 3T3-L1 cells
- Alternative Isoforms of TonEBP with Variable N-termini are Expressed in Mammalian Cells
- Alteration of Hypertonic Responses in Hyperglycemic Human Retinal Pigment Epithelial and Placental Cells