J Korean Ophthalmol Soc.
2014 Aug;55(8):1187-1194.
Transmission Electron Microscopic Findings of Lacrimal Gland Acinar Cells Induced by In Vivo Dry Eye
- Affiliations
-
- 1Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. shadik@yuhs.ac
- 2Morphology Lab., Yonsei Biomedical Research Institute, Seoul, Korea.
- 3Institute of Corneal Dystrophy Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
To determine the change in lacrimal gland (LG) acinar cells induced by in vivo dry eye (DE).
METHODS
Six to 8-week-old (C57BL/6) mice were placed in a controlled environment chamber at <20% humidity for 2 weeks, and a control group was bred in a normal environment. After these 2 weeks of dry eye (DE) induction, the mice were sacrificed and their LGs were collected. Lacrimal gland acinar cell organelle structures were observed with Transmission Electron Microscopy (TEM). TEM images were analyzed using the Image J program.
RESULTS
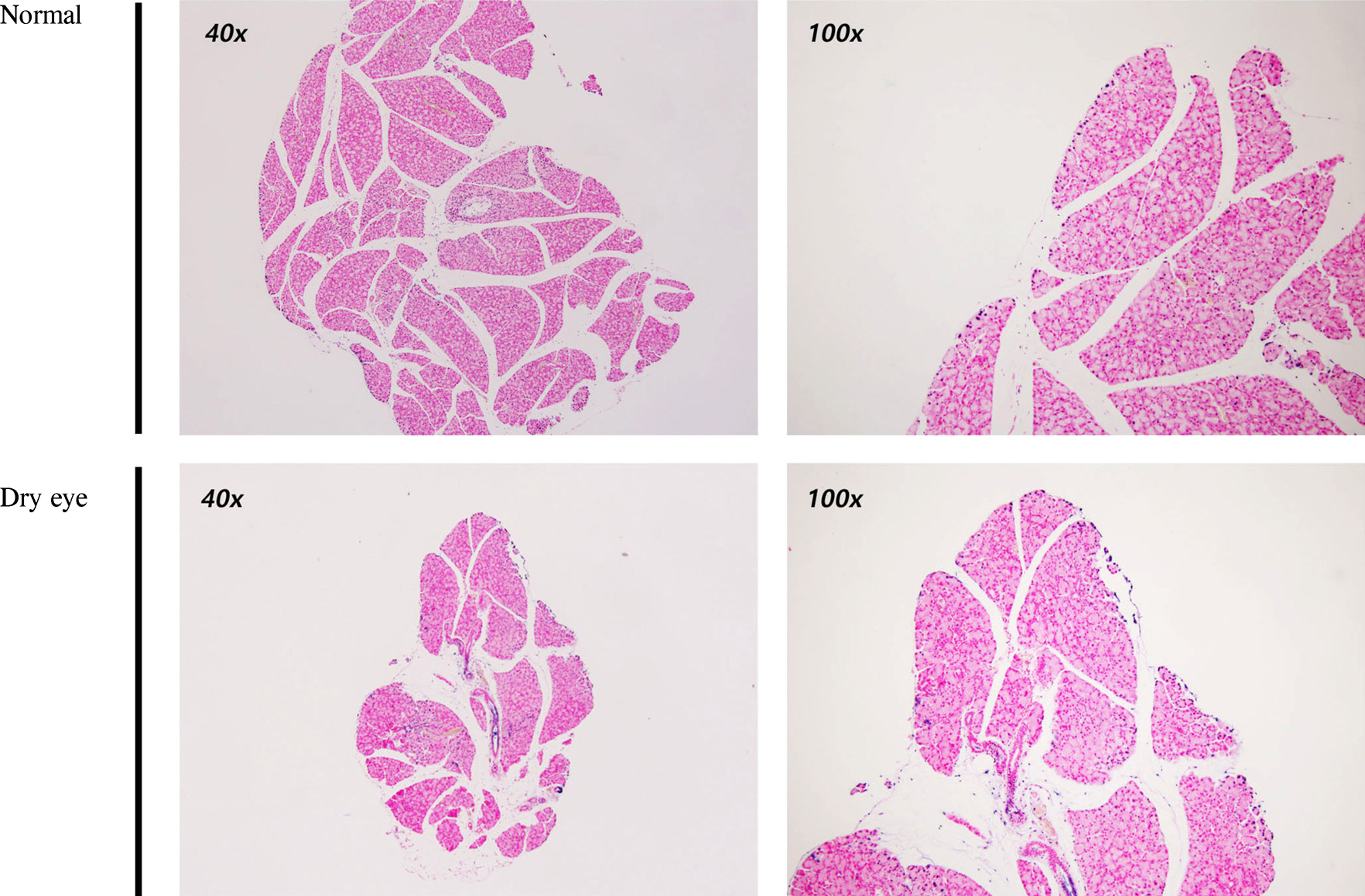
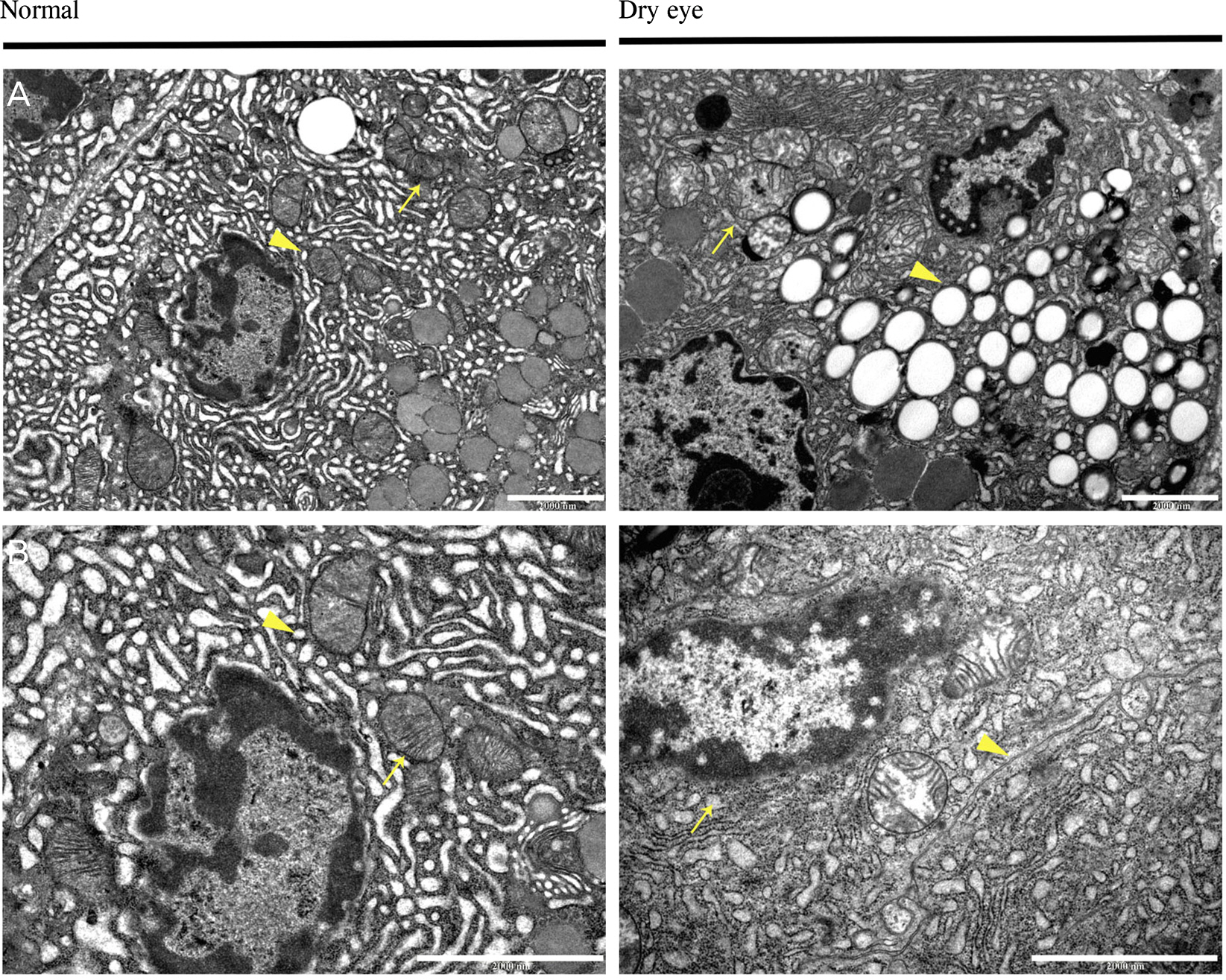
The size of the LGs of DE-induced mice decreased compared to those of normal mice. Terminal deoxynucleotidyl transferase dUTP Nick End Labeling (TUNEL) staining was negative in DE-induced LGs. Under the TEM, the endoplasmic reticulum (ER) lumen was dilated and the lumen density increased in DE-induced mice. Additionally, cell organelles were surrounded by elongated ER lumens. The mitochondrial structure was destroyed and the number of vacuoles increased in the LGs of DE-induced mice.
CONCLUSIONS
Structural changes of the LG developed due to DE induction. This suggests that the detailed mechanisms of these changes were ER stress and autophagy. However, there were no definite signs of apoptosis in the acinar cells of the DE-induced LGs. These findings are regarded as an important clue of the pathogenesis of non-Sjogren-type dry eye.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Stem ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004; 78:409–16.2. Hirayama M, Ogawa M, Oshima M, et al. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013; 4:2497.
Article3. Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006; 66:S102–9.
Article4. Cheng Y, Yang JM. Survival and death of endoplasmic-reticulum-stressed cells: Role of autophagy. World J Biol Chem. 2011; 2:226–31.
Article5. Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005; 115:2656–64.
Article6. Quan W, Jo EK, Lee MS. Role of pancreatic beta-cell death and inflammation in diabetes. Diabetes Obes Metab. 2013; 15(Suppl 3):141–51.7. Fonseca AC, Ferreiro E, Oliveira CR, et al. Activation of the endoplasmic reticulum stress response by the amyloid-beta 1-40 peptide in brain endothelial cells. Biochim Biophys Acta. 2013; 1832:2191–203.
Article8. Deng S, Wang M, Yan Z, et al. Autophagy in retinal ganglion cells in a rhesus monkey chronic hypertensive glaucoma model. PLoS One. 2013; 8:e77100.
Article9. Yung HW, Korolchuk S, Tolkovsky AM, et al. Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 2007; 21:872–84.
Article10. Akamatsu K, Shibata MA, Ito Y, et al. Riluzole induces apoptotic cell death in human prostate cancer cells via endoplasmic reticulum stress. Anticancer Res. 2009; 29:2195–204.11. Uchino Y, Kawakita T, Ishii T, et al. A new mouse model of dry eye disease: oxidative stress affects functional decline in the lacrimal gland. Cornea. 2012; 31(Suppl 1):S63–7.12. Situ P, Simpson TL. Interaction of corneal nociceptive stimulation and lacrimal secretion. Invest Ophthalmol Vis Sci. 2010; 51:5640–5.
Article13. Yun CM, Kang SY, Kim HM, Song JS. Prevalence of dry eye disease among university students. J Korean Ophthalmol Soc. 2012; 53:505–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Concentration of Tear Lysozyme in Normal Subjects and Dry Eye
- Current Concepts and Therapeutic Management of Dry Eye
- Impact of Lacrimal Gland Extraction on the Contralateral Eye in an Animal Model for Dry Eye Disease
- The Change of Thickness of the Lacrimal Gland by Orbital Computed Tomographies associated with Aging
- Age-related Histological Changes of Human Lacrimal Gland in Koreans