J Korean Ophthalmol Soc.
2010 Mar;51(3):423-429.
The Effect of Subconjunctival Injection of Bevacizumab After Resection of Muscle in Rabbit Models
- Affiliations
-
- 1Department of Ophthalmology, Chungbuk National University Hospital, Chungbuk National University School of Medicine, Cheongju, Korea. mychoi@chungbuk.ac.kr
- 2Department of Ophthalmology, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
To study the effects of subconjunctival injection on the conjunctiva and muscles after muscle resection in a rabbit model.
METHODS
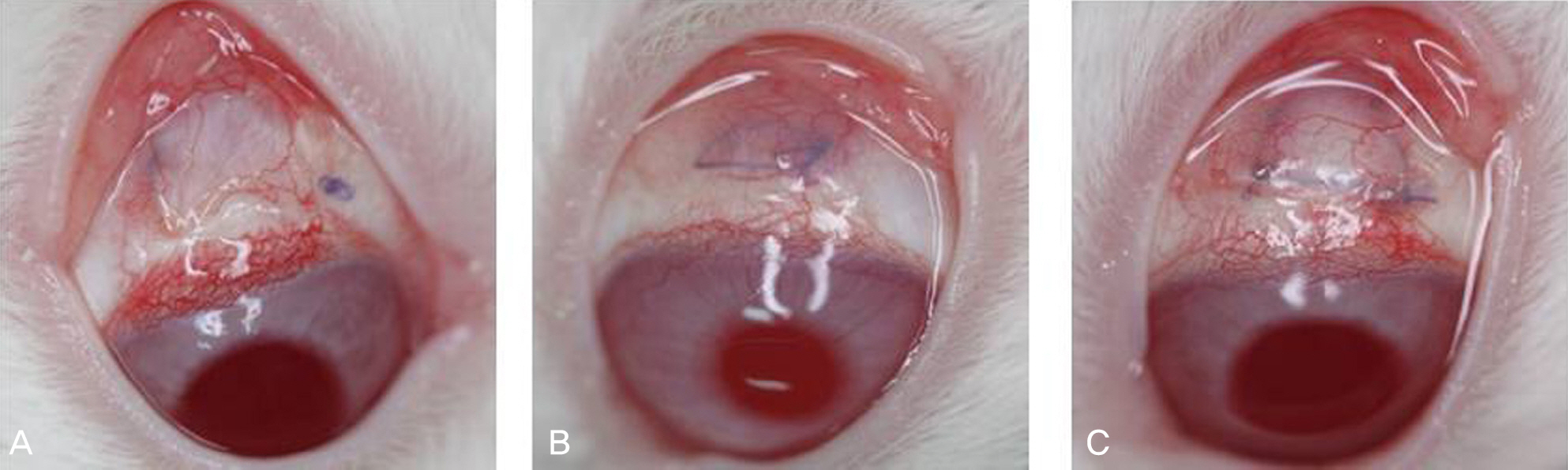
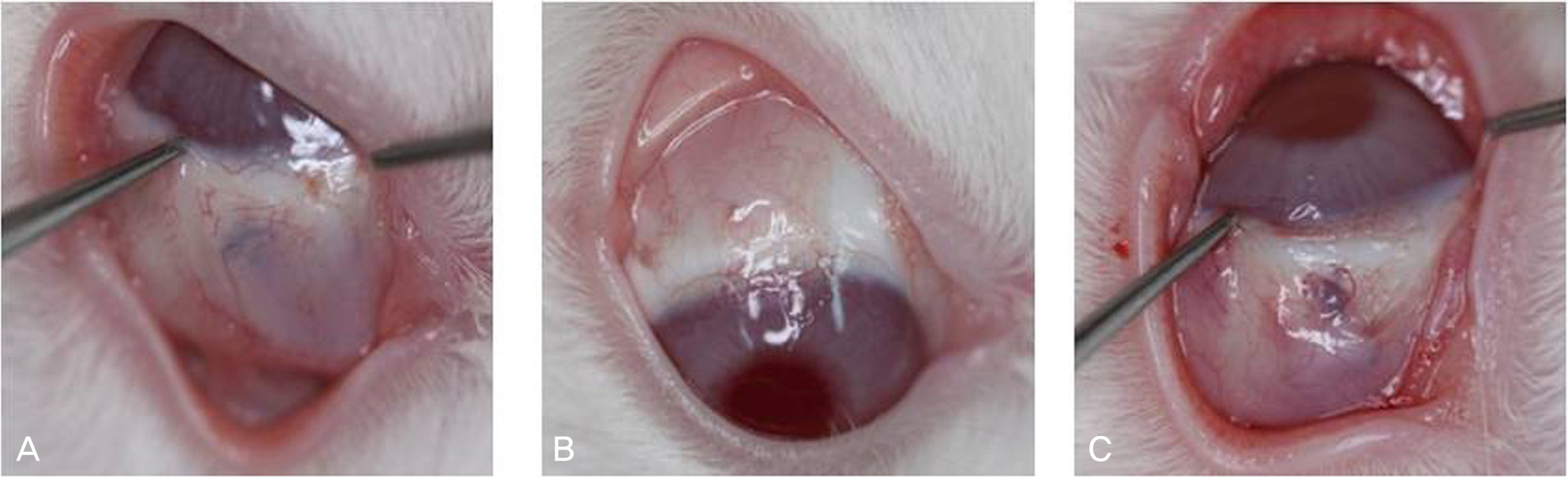
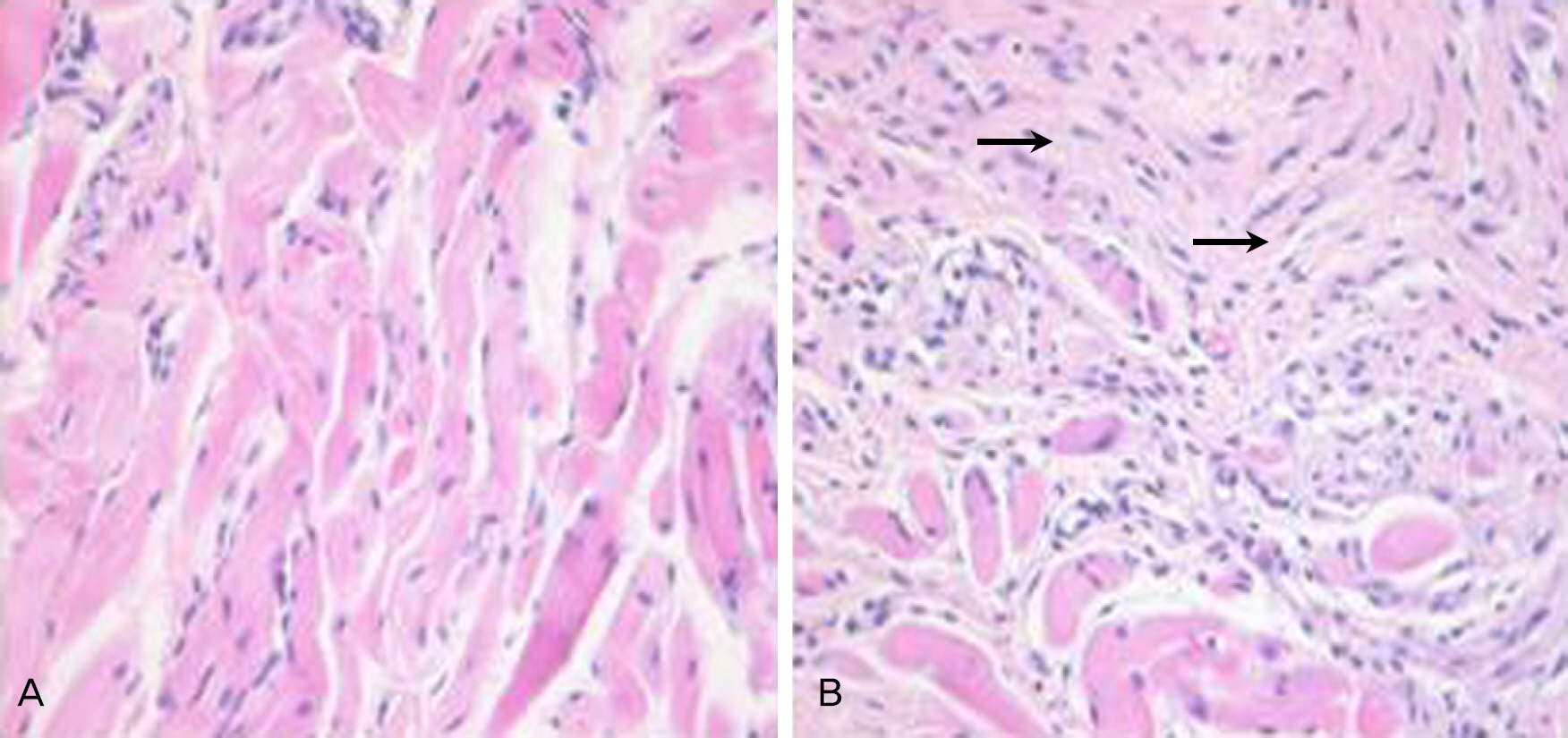
Resection surgery of 5 mm was performed on both the superior rectus muscle (SR) and the inferior rectus muscle (IR) in five white rabbits. As such, 2.5 mg bevacizumab was subconjunctivally injected around the right SR and IR in the experimental group. The left eyes were not injected and were instead used as the control group. The degrees of injection and edema of conjunctiva were classified on a scale from 0 to 4 via gross examination by five examiners at two and four weeks after surgery. The strength of the muscle attachment was assessed, and the degrees of conjunctival inflammation and inflammation and fibrosis of the muscle were classified on a scale from 0 to 4 at four weeks after surgery via histologic examination.
RESULTS
The results of gross examination at two and four weeks after surgery showed positive correlation among the five examiners (k=0.52, k=0.4), although there was no statistically significant difference between the experimental and control groups (p=0.285, p=0.364). There was also no significant difference between the two groups with regard to tensile strength of the attachment (p=0.414), inflammation of the conjunctiva and muscle, or fibrosis of the muscle in histologic examination (p=0.698, p=0.702, p=0.232, respectively).
CONCLUSIONS
There were no significant effects on the inflammation and fibrosis of the conjunctiva or muscles due to subconjunctival injection of 2.5 mg of bevacizumab after muscle resection in a rabbit model.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Wortham E 5th, Anandakrishnan I, Kraft SP, et al. Are antibiotic steroid drops necessary following strabismus surgery? A abdominal, randomized, masked trial. J Pediatr Ophthalmol Strabisumus. 1990; 27:205–7.2. Kim HY, Lee SJ, Lee JH, Lew H. The effect of antibiotics and abdominal drops following strabismus surgery. J Korean Ophthalmol Soc. 1993; 34:327–30.3. Choy AE, Weiss S, Chow C, et al. Dexamethasone sodium abdominal (Decaron) versus saline placebo injections in strabismus surgery. J Pediatr Ophthalmol Strabismus. 1982; 19:140–3.4. von Noorden GK. Principles of surgical treatment. Binocular abdominal and ocular motility. 4th ed. St Louis: CV Mosby;1986. p. 479–531.5. Searl SS, Metz HS, Lindahl KJ. The use of sodium hyaluronate as a biologic sleeve in strabismus surgery. Ann Ophthalmol. 1987; 19:259–62.6. Kim SK, Choi MY. The effects of ADCON®-L on the adhesion abdominal strabismus surgery. J Korean Ophthalmol Soc. 2003; 44:208–14.7. Cho YA, Lee DS, Shon TS. The effect of subconjunctival betha-methasone, 5-fluorouracil and mitomycin C on postoperative abdominal after strabismus surgery. J Korean Ophthalmol Soc. 1994; 35:1698–709.8. Kim CI, Kang SM, Cho YA. The adequate concentration of abdominally injected mitomycin C for the prevention of abdominal adhesion in strabismus. J Korean Ophthalmol Soc. 1998; 39:1271–80.9. Lama PJ, Fechtner RD. Antifibrotics and wound healing in abdominal surgery. Surv Ophthalmol. 2003; 48:314–46.10. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and abdominal of bevacizumab, anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004; 3:391–400.11. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence abdominal findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005; 36:331–5.12. Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab abdominal of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006; 26:383–90.13. Stifter E, Michels S, Prager F, et al. Intravitreal bevacizumab abdominal for neovascular age-related macular degeneration with large submacular hemorrhage. Am J Ophthalmol. 2007; 144:886–92.14. Kim YH, Kim ES, Yu SY, Kwak HW. abdominal effect of abdominal bevacizumab for CNV secondary to age-related macular degeneration. J Korean Ophthalmol Soc. 2008; 49:1935–40.15. Kim JY, Kweon EY, Lee DW, Cho NC. Results of intravitreal bevacizumab for macular edema with retinal vein occlusion and diabetic macular edema. J Korean Ophthalmol Soc. 2008; 49:1275–82.
Article16. Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal abdominal (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006; 26:279–84.17. Yazdani S, Hendi K, Pakravan M. Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma. J Glaucoma. 2007; 16:437–9.
Article18. Lee DW, Lee DG. Two cases of subconjunctival injection to abdominal bleb failure after trabeculectomy. J Korean Ophthalmol Soc. 2008; 49:1345–9.19. Erdurmus M, Totan Y. Subconjunctival bevacizumab for corneal neovascularization. Grafes Arch Clin Exp Ophthalmol. 2007; 245:1577–9.
Article20. Bahar I, Kaiseman I, McAllum P, et al. Subconjunctival abdominal injection for corneal neovascularization. Cornea. 2008; 27:142–7.21. Munton CG. Tissue adhesive in ocular surgery: a prospective study. Exp Eye Res. 1971; 11:1–6.
Article22. Ozkan SB, Kir E, Culhaci N, Dayanir V. The effect of Seprafilm on adhesion in strabismus surgery-An experimental study. J AAPOS. 2004; 8:46–9.23. Lee JW, Park YJ, Kim IT, Lee KW. Clinical results after application of bevacizumab in recurrent pterygium. J Korean Ophthalmol Soc. 2008; 49:1901–9.
Article24. Weijtens O, Feron EJ, Schoemaker RC, et al. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am J Ophthalmol. 1999; 128:192–7.
Article25. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of abdominal bevacizumab (Avastin). Ophthalmology. 2007; 114:855–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recurrent Conjunctival Granuloma Treated with Subconjunctival Bevacizumab
- The Effect of Subconjunctival Bevacizumab Injection after Primary Pterygium Surgery
- Effects of Subconjunctival Bevacizumab Injection after Primary Pterygium Surgery
- Clinical Results After Application of Bevacizumab in Recurrent Pterygium
- Bi-weekly Subconjunctival Injection of Bevacizumab for Corneal Neovascularization after Burn Injury