J Korean Orthop Assoc.
2011 Oct;46(5):357-363.
Enhancement of Vasculogenesis and Osteogenesis Using Granulocyte-Colony Stimulating Factor in the Rat Model of Tibial Distraction Osteogenesis
- Affiliations
-
- 1Department of Orthopaedic Surgery, Seoul National University College of Medicine, Seoul, Korea. leedy@snu.ac.kr
- 2Department of Orthopaedic Surgery, Jeju National University College of Medicine, Jeju, Korea.
Abstract
- PURPOSE
Proper speed of distraction is critical for successful new bone formation in distraction osteogenesis. The purpose of this study was to evaluate the effect of granulocyte-colony stimulating factor (G-CSF) on formation of new blood vessels and new bones in the rat model of tibial distraction osteogenesis (DO) to develop enhancement method of bone formation while increasing the distraction speed.
MATERIALS AND METHODS
Forty two rat-tibial DO models were included in this study, and were divided into 3 groups; group I (rapid distraction), group II (rapid distraction with G-CSF), and group III (slow distraction). The amount of bone formation and relative blood flow were analyzed by sequential radiographs and laser Doppler perfusion imaging (LDPI). Blood sampling was done before G-CSF injection, at 2 weeks and 5 weeks after G-CSF injection and surface expression such as Scal-1+ and C-kit+ of endothelial progenitor cells (EPCs) was analyzed by fluorescence-activated cell sorter (FACS) for the effects of G-CSF in inducing mobilization of EPCs.
RESULTS
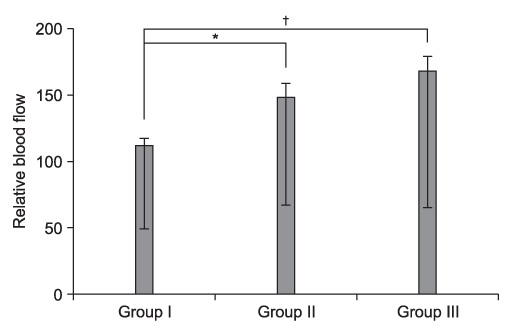
The amount of new bone formation in the distraction gap on serial radiographs was higher during the consolidation period in groups II and III than in group I but, the difference was not significant (p>0.05). The relative blood flow in the distraction gap in groups II and III increased more significantly than in group I (p<0.05). FACS analysis showed an increased EPCs fraction after G-CSF injection.
CONCLUSION
We demonstrated that G-CSF administration ameliorated bone formation and blood flow during rapid distraction in the rat model of tibial distraction osteogenesis. We think that G-CSF has an effect on mobilization of EPCs resulting in an increase in the blood flow.
Keyword
MeSH Terms
Figure
Reference
-
1. Choi IH, Chung CY, Cho TJ, Yoo WJ. Angiogenesis and mineralization during distraction osteogenesis. J Korean Med Sci. 2002. 17:435–447.
Article2. Lee DY, Cho TJ, Lee HR, et al. Distraction osteogenesis induces endothelial progenitor cell mobilization without inflammatory response in man. Bone. 2010. 46:673–679.
Article3. Lee DY, Cho TJ, Kim JA, et al. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone. 2008. 42:932–941.
Article4. Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989. 238:249–281.5. Carvalho RS, Einhorn TA, Lehmann W, et al. The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone. 2004. 34:849–861.
Article6. Choi IH, Ahn JH, Chung CY, Cho TJ. Vascular proliferation and blood supply during distraction osteogenesis: a scanning electron microscopic observation. J Orthop Res. 2000. 18:698–705.
Article7. Aronson J. Limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method. J Bone Joint Surg Am. 1997. 79:1243–1258.8. Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989. 239:263–285.9. Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999. 85:221–228.
Article10. Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999. 5:434–438.
Article11. Stroncek DF, Clay ME, Herr G, et al. The kinetics of G-CSF mobilization of CD34+ cells in healthy people. Transfus Med. 1997. 7:19–24.
Article12. Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001. 98:10344–10349.
Article13. Fang TD, Salim A, Xia W, et al. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res. 2005. 20:1114–1124.
Article14. Chappard D, Retailleau-Gaborit N, Legrand E, Baslé MF, Audran M. Comparison insight bone measurements by histomorphometry and microCT. J Bone Miner Res. 2005. 20:1177–1184.15. Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002. 99:9656–9661.
Article16. Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009. 24:1347–1353.
Article17. Riddle RC, Khatri R, Schipani E, Clemens TL. Role of hypoxia-inducible factor-1alpha in angiogenic-osteogenic coupling. J Mol Med (Berl). 2009. 87:583–590.18. Körbling M, Reuben JM, Gao H, et al. Recombinant human granulocyte-colony-stimulating factor-mobilized and apheresis-collected endothelial progenitor cells: a novel blood cell component for therapeutic vasculogenesis. Transfusion. 2006. 46:1795–1802.
Article19. Cho HJ, Kim TY, Cho HJ, et al. The effect of stem cell mobilization by granulocyte-colony stimulating factor on neointimal hyperplasia and endothelial healing after vascular injury with bare-metal versus paclitaxel-eluting stents. J Am Coll Cardiol. 2006. 48:366–374.
Article20. Powell TM, Paul JD, Hill JM, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005. 25:296–301.
Article21. Aronson J, Shen XC, Skinner RA, Hogue WR, Badger TM, Lumpkin CK Jr. Rat model of distraction osteogenesis. J Orthop Res. 1997. 15:221–226.
Article22. Takeshita S, Pu LQ, Stein LA, et al. Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation. 1994. 90:II228–II234.23. Fadini GP, Sartore S, Schiavon M, et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006. 49:3075–3084.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chronic Osteomyelitis in Distraction Osteogenesis Area of Tibial Shaft: A Case Report
- Case reports of antero-posteior movement with distraction osteogenesis in maxillary anterior segment
- Sweet Syndrome in a Child with Aplastic Anemia after Receiving Recombinant Granulocyte Colony-stimulating Factor
- Two cases of congenital agranulocytosis treated with recombinant human granulocyte colony-stimulating factor
- The effect of granulocyte colony-stimulating factor in chemotherapy of acute myelogenous leukemia