J Korean Soc Clin Pharmacol Ther.
2012 Dec;20(2):135-144.
Comparison of Pharmacokinetics and Safety of Two Formulations of Letrozole (2.5 mg) in Healthy Male Volunteers
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, Seoul, Korea. mdlhs@amc.seoul.kr
- 2Department of Clinical Pharmacology and Therapeutics, University of Ulsan College of Medicine, Seoul, Korea.
- 3Pharmacokinetic and Pharmacogenetic Laboratory, Clinical Trial Center, Asan Medical Center, Seoul, Korea.
- 4Department of Pharmacology and Clinical Pharmacology & Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Korea.
- 5Clinical Trials Center, Pusan National University Hospital, Busan, Korea.
- 6Department of Clinical Pharmacology, Busan Paik Hospital, Busan, Korea.
- 7Department of Clinical Pharmacology and Therapeutics, Samsung Medical Center, Seoul, Korea.
Abstract
- BACKGROUND
Letrozole is an oral non-steroidal inhibitor of the aromatase enzyme, which has proven to be a useful drug against breast cancer.
METHODS
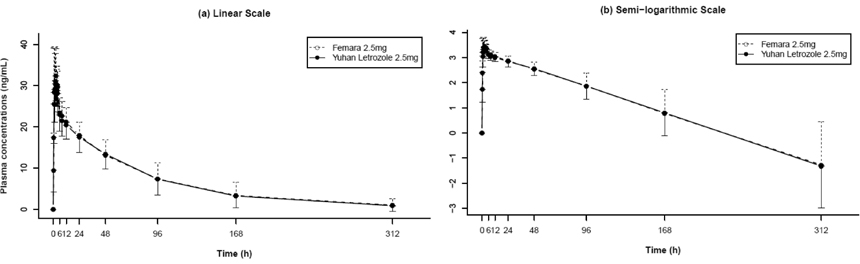
This single-dose, randomized 2 x 2 crossover study was conducted in healthy male volunteers. Participants of each sequence group (each 13 volunteers for sequence group) received, in randomized sequence, a single oral 2.5-mg dose of generic letrozole (test) or branded letrozole (reference). Each treatment period was separated by a 5-week washout period. Blood samples were collected for up to 312 hours after drug administration, and drug concentrations were determined using validated LC/MS-MS. Pharmacokinetic properties were obtained using noncompartmental analysis. Drug tolerability was assessed throughout the study, using measurements of vital signs, physical examination, clinical chemistry testing, EKG, and interviews.
RESULTS
A total of 26 subjects completed the study. The geometric mean ratios (90% CI) of Cmax and AUClast were 0.92 (0.85 - 0.99) and 1.01 (0.97 - 1.04), respectively. No serious AEs were reported, and there were no clinically significant differences between test and reference groups.
CONCLUSION
The findings from this study suggest bioequivalence between two formulations of letrozole in healthy male volunteers. The safety profile of two formulations had similar characteristics.
Keyword
MeSH Terms
Figure
Reference
-
1. Campos SM. Aromatase inhibitors for breast cancer in postmenopausal women. Oncologist. 2004. 9(2):126–136.
Article2. Dellapasqua S, Colleoni M. Letrozole. Expert Opin Drug Metab Toxicol. 2010. 6(2):251–259.
Article3. KFDA. Korea good clinical practice (KGCP) guidelines. last visited on 07 July 2012. http://clinicaltrials.kfda.go.kr/guide/laws/board_list.jsp?category_seq=12 [Online].4. Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects. last visited on 07 July 2012. http://www.wma.net/en/30publications/10policies/b3/ [Online].5. Marfil F, Pineau V, Sioufi A, Godbillon SJ. High-performance liquid chromatography of the aromatase inhibitor, letrozole, and its metabolite in biological fluids with automated liquid-solid extraction and fluorescence detection. J Chromatogr B Biomed Appl. 1996. 683(2):251–258.
Article6. Zarghi A, Foroutan SM, Shafaati A, Khoddam A. Quantification of carvedilol in human plasma by liquid chromatography using fluorescence detection: application in pharmacokinetic studies. J Pharm Biomed Anal. 2007. 44(1):250–253.
Article7. Rowland M, Tozer TN. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. 2011. 4th ed. Wolters, Kluwer/Lippincott, Williams & Wilkins.8. KFDA. Bioequivalence guideline in Korea. last visited on July 07 2012. Published by Korea Food and Drug Administration;http://www.kfda.go.kr/index.kfda?mid=226&pageNo=1&seq=139&cmd=v [Online].9. Lonning P, Pfister C, Martoni A, Zamagni C. Pharmacokinetics of third-generation aromatase inhibitors. Semin Oncol. 2003. 30:4 Suppl 14. 23–32.
Article10. Noh YH, Ko YJ, Cho SH, Ghim JL, Choe S, Jung JA, Kim UJ, Jin SJ, Park HJ, Song GS, Lim HS. Pharmacokinetic comparison of 2 formulations of anastrozole (1 mg) in healthy Korean male volunteers: a randomized, single-dose, 2-period, 2-sequence, crossover study. Clin Ther. 2012. 34(2):305–313.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Pharmacokinetic Characteristics and Safety Between JW Amlodipine(R) Tablet 5 mg and Novarsc(R) Tablet 5 mg in Healthy Male Volunteers
- Bioequivalence of the pharmacokinetics between two formulations of 0.2 mg tamsulosin hydrochloride in healthy subjects
- Pharmacokinetics comparison of solifenacin tartrate and solifenacin succinate: a randomized, open-label, single-dose, 2-way crossover study in healthy male volunteers
- Comparative pharmacokinetic and tolerability evaluation of two simvastatin 20 mg formulations in healthy Korean male volunteers
- Pharmacokinetic comparison of two levofloxacin 100-mg tablet formulations and determination of time point appropriately reflecting its area under the curve