J Korean Diabetes Assoc.
2006 Jan;30(1):39-46.
A Study on Insulin action using fibroblast of leprechaunism patients
- Affiliations
-
- 1Department of Pediatrics, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, Korea.
- 2Clinical Research, Samsung Biomedical Research Institute, Seoul, Korea.
- 3Division of Molecular Life Sciences, Center for Cell Signaling Research, Ewha Womans University, Seoul, Korea.
Abstract
-
BACKGROUND: Leprechaunism, which is caused by insulin receptor defect, is clinically characterized by hyperinsulinemia, stunted growth, dysmorphic somatic abnormalities, lipodystrophy and early death around 2-3 years of age.
METHODS
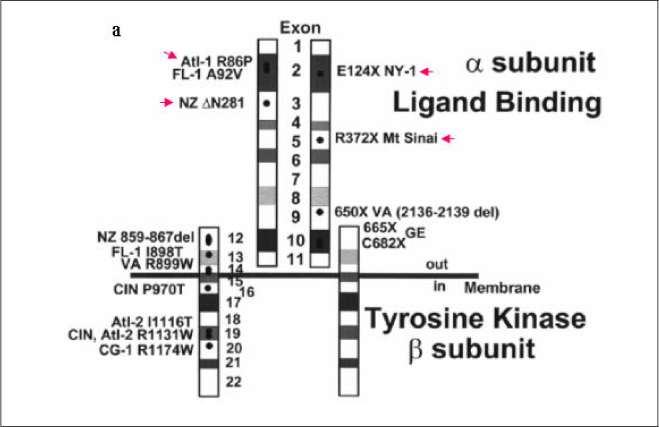
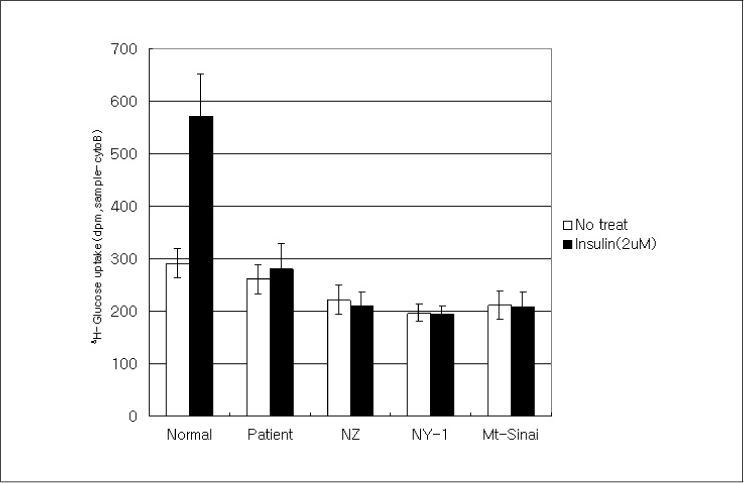
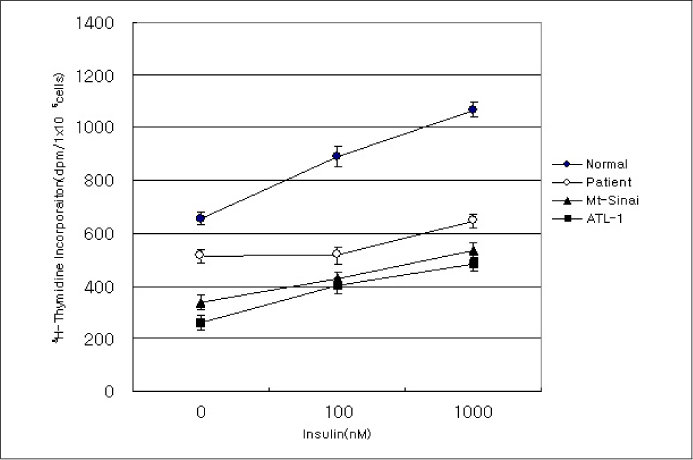
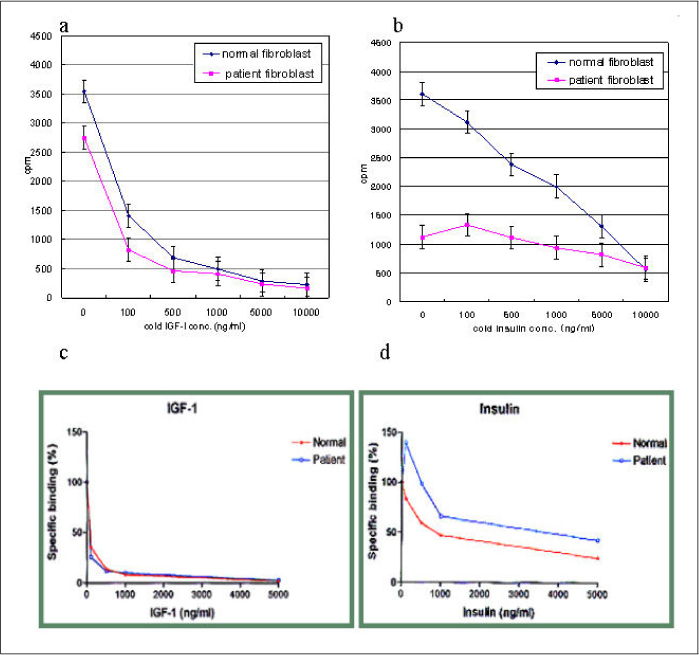
In this study we describe 2 cases of Korean leprechaunism patients. In addition, the disturbed insulin induced effects such as glucose uptake, insulin binding assay, thymidine uptake and PDGF stimulated phosphorylation change were investigated using the fibroblasts from 1 Korean patient and those from 4 foreign leprechaunism patients.
RESULTS
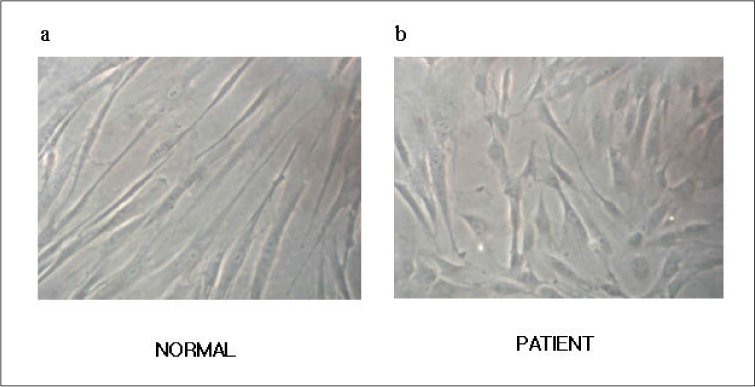
The morphologic study of fibroblasts derived from patient suggests that the fibroblasts of patient are less differentiated than the fibroblasts of control. Moreover, insulin induced pathways as well as PDGF stimulated phosphorylation change were disturbed in the fibroblasts of the patient.
CONCLUSION
Our results suggest that the signal transduction in the fibroblasts of leprechaunism is reduced not only by insulin stimuli but also by other growth factors like PDGF.
MeSH Terms
Figure
Reference
-
1. Cohen P, Nimmo HG, Proud CG. How does insulin stimulate glycogen synthesis? Biochem Soc Symp. 1978. 43:69–95.2. Olefsky JM, Ciaraldi TP, Kolterman OG. Mechanisms of insulin resistance in non-insulin-dependent (type II) diabetes. Am J Med. 1985. 79:12–22.3. Lyen KR. The insulin receptor. Ann Acad Med Singapore. 1985. 14:364–373.4. Tan MH, Bonen A. Insulin receptors: their roles in the pathogenesis and management of type II diabetes mellitus. Ann Acad Med Singapore. 1985. 14:360–363.5. Takahashi Y, Tobe K, Kadowaki H, Katsumata D, Fukushima Y, Yazaki Y, Akanuma Y, Kadowaki T. Roles of insulin receptor substrate-1 and Shc on insulin-like growth factor I receptor signaling in early passages of cultured human fibroblasts. Endocrinology. 1997. 138:741–750.6. Tsujino G, Yoshinaga T. A case of leprechaunism and an analysis of some clinical manifestations of this syndrome. Z Kinderheilk. 1975. 118:347–445.7. Longo N, Wang Y, Smith SA, Langley SD, DiMeglio LA, Giannella-Neto D. Genotype-phenotype correlation in inherited severe insulin resistance. Hum Mol Genet. 2002. 11:1465–1475.8. Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, Jose PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat genet. 1996. 12:106–109.9. Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000. 6:87–97.10. Yamauchi T, Tobe K, Tamemoto H, Ueki K, Kaburagi Y, Yamamoto-Honda R, Takahashi Y, Yoshizawa F, Aizawa S, Akanuma Y, Sonenberg N, Yazaki Y, Kadowaki T. Insulin signaling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1 deficient mice. Mol Cell Biol. 1996. 16:3074–3084.11. Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997. 88:561–572.12. Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature. 1995. 377:151–155.13. Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998. 2:559–569.14. Garg A, Wilson R, Barnes R, Arioglu E, Zaidi Z, Gurakan F, Kocak N, O'Rahilly S, Taylor SI, Patel SB, Bowcock AM. A gene for congenital general lipodystrophy maps to human chromosome 9q34. J Clin Endocrinol Metab. 1999. 84:3390–3394.15. Nakae J, Kato M, Murashita M, Shinohara N, Tajima T, Fujieda K. Long-term effect of recombinant human insulin-like growth factor I on metabolic and growth control in a patient with leprechaunism. J Clin Endocrinol Metab. 1998. 83:542–549.16. Takahashi Y, Tobe K, Kadowaki H, Katsumata D, Fukushima Y, Yazaki Y, Akanuma Y, Kadowaki T. Roles of insulin receptor substrate-1 and Shc on insulin-like growth factor I receptor signaling in early passages of cultured human fibroblasts. Endocrinology. 1997. 138:741–750.