J Korean Diabetes Assoc.
2006 Jan;30(1):25-38.
The long-term effect of ramipril on Gialpha2-protein and Protein Tyrosine Phosphatase 1B in an animal model of type 2 diabetes(OLETF rat)
- Affiliations
-
- 1Division of Endocrinology & Metabolism, Department of Internal medicine, The Catholic University of Korea, Seoul, Korea.
- 2Catholic Research Institutes of medical Science, The Catholic University of Korea, Seoul, Korea.
Abstract
-
BACKGROUND: The regulation of tyrosine phosphorylation/dephosphorylation is an important mechanism in various intracellular metabolism. Also impaired insulin signal transduction is important in pathogenesis of type 2 diabetes. It has been reported that PTP1B is a negative regulator of insulin action, and Gialpha2-protein is related to the regulation of PTP1B. Herein we investigated the long-term effects of ramipril on PTP1B/insulin signal protein interaction and the relation between Gialpha2 and PTP1B in animal model of type 2 diabetes (OLETF rat).
METHODS
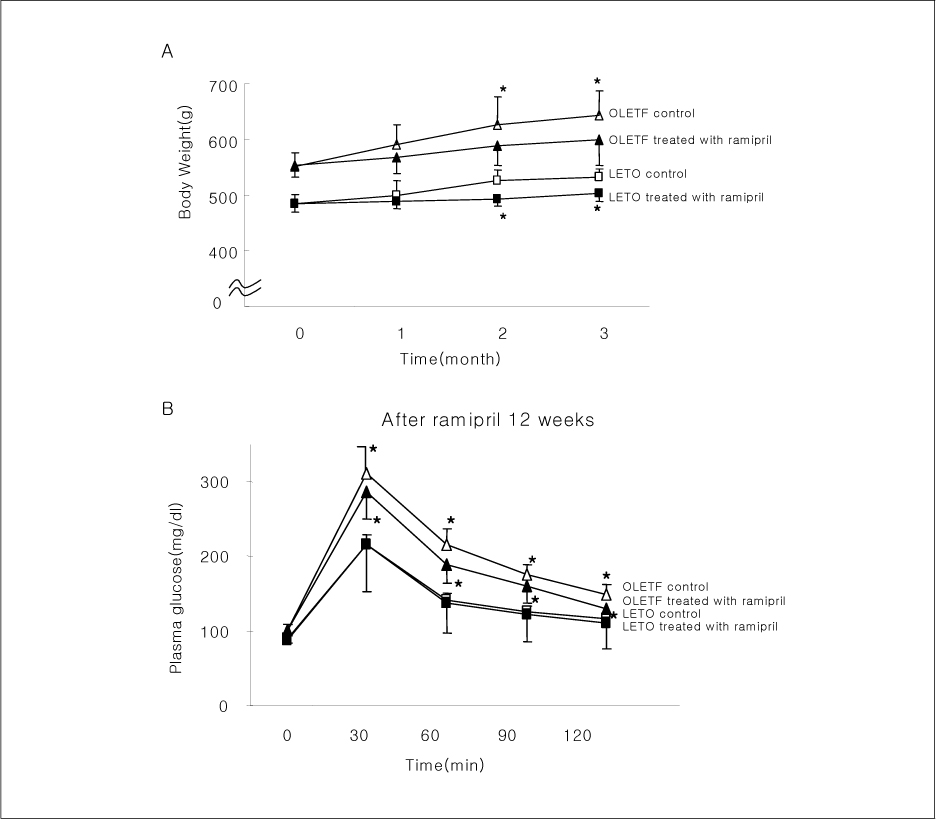
OLETF rats and age-matched LETO rats were divided into two groups. One group of rats received ramipril (10 mg/kg body weight) for 12 weeks, and another group did not. Finally, each group was divided into 2 subgroups, with or without insulin injection intravenously, before sacrifice. After sacrifice, tissues extracts of liver, hind limb muscle, and epididymal fat were obtained for quantification of PTP1B, Gialpha2, and several insulin signal proteins by western blotting.
RESULTS
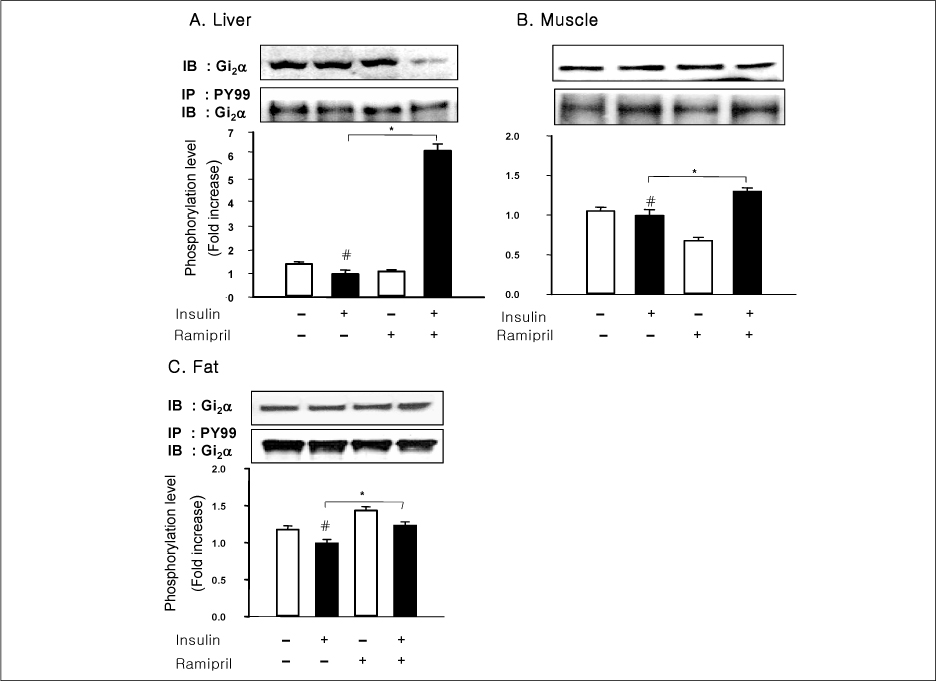
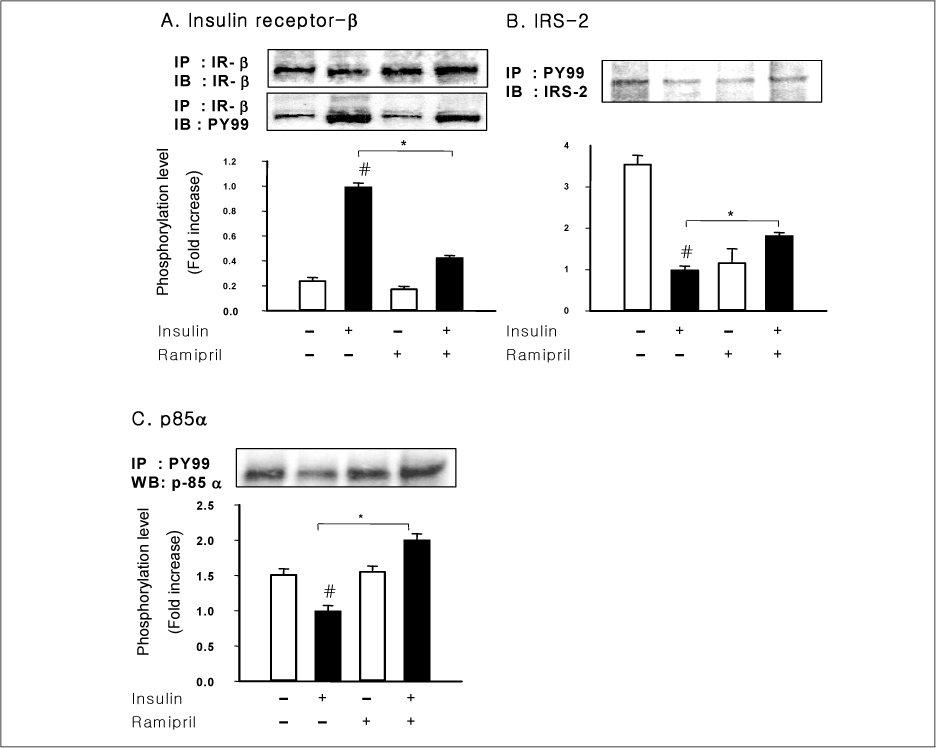
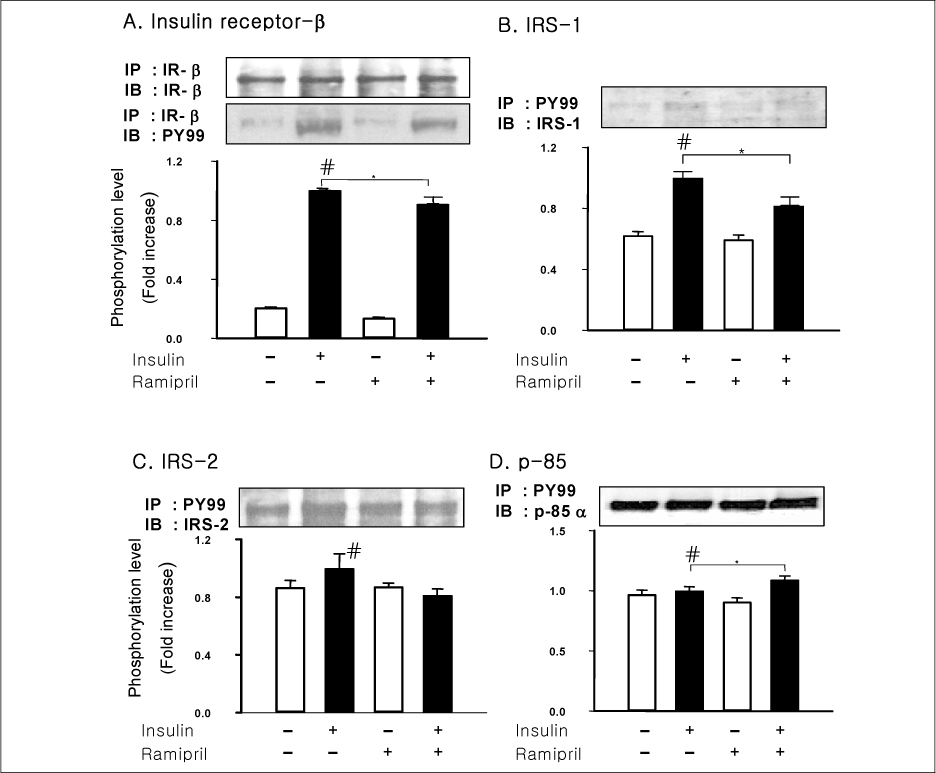
In liver and muscle, the levels of basal PTP1B and activated PTP1B of OLETF rats treated with ramipril and insulin were significantly decreased. The levels of Gialpha2, activated IRS-2, and activated p-85alpha were significantly increased in OLETF rats treated with ramipril and insulin. In adipose tissue, the levels of Gialpha2 and activated p-85alpha of OLETF rats treated with ramipril and insulin were slightly increased as in liver and muscle. But, the levels of basal PTP1B and activated PTP1B were significantly increased. And, the levels of activated IRS-1 and activated IRS-2 were decreased.
CONCLUSION
These results suggest that the improvement of insulin sensitivity by treatment with ramipril was related to the decreased level of activated PTP1B. Also, we could suggest that the changes of activated PTP1B level was related with the changes of Gialpha2-protein. However, the results of adipose tissue were different from those of liver and muscle. So it seemed likely that there would be various major modulators for regulation of insulin signal pathway according to tissue.
Keyword
MeSH Terms
-
Adipose Tissue
Animals*
Blotting, Western
Extremities
Insulin
Insulin Resistance
Liver
Metabolism
Models, Animal*
Protein Tyrosine Phosphatase, Non-Receptor Type 1*
Protein Tyrosine Phosphatases*
Ramipril*
Rats
Rats, Inbred OLETF
Signal Transduction
Tyrosine
Insulin
Protein Tyrosine Phosphatase, Non-Receptor Type 1
Protein Tyrosine Phosphatases
Ramipril
Tyrosine
Figure
Reference
-
1. Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988. 37:1595–1607.2. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Inv. 2000. 106:473–481.3. Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001. 104:517–529.4. White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994. 269(1):1–4.5. Goldstein BJ. Regulation of insulin receptor signaling by protein-tyrosine dephosphorylation. Receptor. 1993. 3:1–15.6. Kenner KA, Anyanwu E, Olefsky JM, Kusari J. Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J Biol Chem. 1996. 271:19810–19816.7. Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M, Cheung AT, Kolls JK, Kikkawa R, Kashiwagi A. Protein-tyrosine phosphatase 1B negatively regulates insulin signaling in L6 myocytes and Fao hepatoma cells. J Biol Chem. 2001. 276:10207–10211.8. Gum RJ, Gaede LL, Koterski SL, Heindel M, Clampit JE, Zinker BA, Trevillyan JM, Ulrich RG, Jirousek MR, Rondinon CM. Reduction of protein tyrosine phosphatase 1B increase insulin-dependent signaling in ob/ob mice. Diabetes. 2003. 52:21–29.9. Zabolotny JM, Haj FG, Kim HJ, Shulman GI, Kim JK, Neel BG, Kahn BB. Transgenic overexpression of PTP1B in muscle causes insulin resistance but overexpression with LAR does not additively impair insulin action. J Biol Chem. 2004. 279:24844–24851.10. Ahmad F, Li PM, Meyerovitch J, Goldstein BJ. Osmotic loading of neutralizing antibodies demonstrates a role for protein-tyrosine phosphatase 1B In negative regulation of the Insulin action pathway. J Biol Chem. 1995. 270:20503–20508.11. Kushner JA, Haj FG, Klaman LD, Dow MA, Kahn BB, Neel BG, White MF. Islet-sparing effects of protein tyrosine phosphatase-1b deficiency delays onset of diabetes in IRS2 Knockout mice. Diabetes. 2004. 53:61–66.12. Tao J, Malbon CC, Wang HY. Giα2 enhances insulin signaling via suppression of protein-tyrosine phosphatase 1B. J Biol Chem. 2001. 276:39705–39712.13. Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, Luomanmaki K, Dahlof B, de Faire U, Morlin C, Karlberg BE, Wester PO, Bjorck JE. Effects of angiotensin-converting-enzyme inhibition compare with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project(CAPP) randomized trial. Lancet. 1999. 353:611–616.14. Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel BH, Zinman B. Ramipril and the development of diabetes. JAMA. 2001. 286:1882–1885.15. Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertensive study(LIFE): a randomized trial against atenolol. Lancet. 2002. 359:995–1003.16. RAO RH. Pressor doses of angiotensin II increase hepatic glucose output and decrease insulin sensitivity in rats. J Endocrinol. 1996. 148(2):311–318.17. Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Katagiri H, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Komuro I, Fujita T. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2002. 40:872–879.18. Krutzfeldt J, Raasch W, Klein HH. Ramipril increases the protein level of skeletal muscle IRS-1 and alters protein tyrosine phosphatase activity in spontaneously hypertensive rats. Arch Pharmacol. 2000. 362:1–6.19. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–256.20. Moxham CM, Malbon CC. Insulin action impaired by deficiency of the G-protein subunit Giα2. Nature. 1996. 379:840–844.21. Nawano M, Anai M, Funaki M, Kobayashi H, Kanda A, Fukushima Y, Inukai K, Ogihara T, Sakoda H, Onishi Y, Kikuchi M, Yazaki Y, Oka Y, Asano T. Imidapril, an angiotensin-converting enzyme inhibitor, improves insulin sensitivity by enhancing signal transduction via insulin receptor substrate proteins and improving vascular resistance in the Zuker Fatty rat. Metabolism. 1999. 48:1248–1255.22. Sechi LA, Griffin CA, Zingaro L, Valentin JP, Bartoli E, Schambelan M. Effects of angiotensin II on insulin receptor binding and mRNA levels in normal and diabetic rats. Diabetologia. 1997. 40:770–777.23. Ludvik B, Kueenburg E, Brunnbauer M, Schernthaner G, Prager R. The effects of ramipril on glucose tolerance, insulin secretion, and insulin sensitivity in patients with hypertension. J Cardiovasc Pharmacol. 1991. 18:Suppl 2. S157–S159.24. Ukkola O, Santaniemi M. Protein tyrosine phosphatase 1B: a new target for the treatment of obesity and associated co-morbidities. Int Med. 2002. 251:467–475.25. Tao J, Malbon CC, Wang HY. Insulin stimulates tyrosine phosphorylation and inactivation of protein-tyrosine phosphatase 1B in vivo. J Biol Chem. 2001. 276:29520–29525.26. Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramchandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase 1B gene. Science. 1999. 283:1544–1548.27. Heyworth CM, Houslay MD. Insulin exerts action through a distinct species of guanine nucleotide regulatory protein : inhibition of adenylate cyclase. Biochem J. 1983. 214:547–552.28. Gawler D, Milligan G, Spiegel AM, Unson CG, Houslay MD. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987. 327:229–232.29. Crespo P, de Mora JF, Aaronson DS, Santos E, Gutkind JS. Transforming G protein-coupled receptors block insulin and ras-induced adipocytic differentiation in 3T3-L1 cells: evidence for a PKC and MAP kinase independent pathway. Biochem Biophys Res Commun. 1998. 245:554–561.30. Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998. 280:2112–2114.31. Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998. 273:18677–18680.32. Wang L, Hayashi H, Kishi K, Huang L, Hagi A, Tamaoka K, Hawkins PT, Ebina Y. Gi-mediated translocation of GLUT4 is independent of p85/p110alpha and p110gamma phosphoinositide 3-kinases but might involve the activation of Akt kinase. Biochem J. 2000. 345:543–555.33. Zheng XL, Guo J, Wang H, Malbon CC. Expression of constitutively activated Gialpha2 in vivo ameliorates streptozotocin-induced diabetes. J Biol Chem. 1998. 273:23649–23651.34. Pandey SK, Anand-Srivastava MB. Modulation of G-protein expression by the angiotensin converting enzyme inhibitor captopril in heart from spontaneously hypertensive rats. AJH. 1996. 9:833–837.35. Calera MR, Vallega G, Pilch PF. Dynamics of protein-tyrosine phosphatase In rat adipocytes. J Biol Chem. 2000. 275:6308–6312.36. He W, Craparo A, Zhu Y, O'Neill TJ, Wang LM, Pierce JH, Gustafson TA. Interaction of insulin receptor substrate-2(IRS-2) with the insulin and insulin-like growth factor I receptor. Evidence for two distinct phosphotyrosine-dependent interaction domains within IRS-2. J Biol Chem. 1996. 271:11641–11648.37. Sawka-Verhelle D, Tartare-Deckert S, White MF, Van Obberghen E. Insulin receptor substrate-2 binds to the insulin receptor through its phosphotyrosine-binding domain and through a newly identified domain comprising amino acids 591-786. J Biol Chem. 1996. 271:5983–5989.38. Ogihara T, Shin BC, Anai M, Katagiri H, Inukai K, Funaki M, Fukushima Y, Ishihara H, Takata K, Kikuchi M, Yazak Y, Oka Y, Asano T. Insulin receptor substrate(IRS)-2 is dephosphorylated more rapidly than IRS-1 via its association with phosphatidylinositol 3-kinase in skeletal muscle cells. J Biol Chem. 1997. 272:12868–12873.39. Rother KI, Imai Y, Caruso M, Beguinot F, Formisano P. Evidence that IRS-2 phosphorylation is required for insulin action in hepatocytes. J Biol Chem. 1988. 273:17491–17497.40. Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000. 49:1880–1889.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interaction between chicken protein tyrosine phosphatase 1 (CPTP1)-like rat protein phosphatase 1 (PTP1) and p60v-src in v-src-transformed Rat-1 fibroblasts
- The Effect of Long-term Treatment of Ramipril on Glucose Tolerance and Pancreatic Islets in Type 2 Diabetes Animal Model (OLETF Rats)
- Opening of ATP-sensitive K+ channel by pinacidil requires serine/threonine phosphorylation in rat ventricular myocytes
- Angiotensin-Converting Enzyme Inhibitor Ramipril Improves Coronary Microvascular Remodeling in Type II Diabetic Rats
- Regulation of chicken protein tyrosine phosphatase 1 and human protein tyrosine phosphatase 1B activity by casein kinase II- and p56lck-mediated phosphorylation