J Korean Soc Hypertens.
2012 Sep;18(3):88-96.
Pharmacokinetic and Pharmacodynamic Study Determines Factors Affecting Blood Pressure Response to Valsartan
- Affiliations
-
- 1Department of Cardiology, Kyung Hee University Hospital at Gangdong, Seoul, Korea. cardio-cho@daum.net
- 2Department of Clinical Pharmacology, Kyung Hee University Hospital, Seoul, Korea.
Abstract
- BACKGROUND
Valsartan is an angiotensin II receptor blocker and is used for patient with hypertension. Although response to valsartan varies each individual, there is no study about factors affecting the variability of valsartan response.
METHODS
To investigate the effects of valsartan on the baseline characteristics of blood pressure, single group, open label, pre- and post-comparison clinical study was conducted. Total 21 male Korean volunteers were enrolled. Each subject was administered no drugs in first period and valsartan 80 mg (Diovan HCT) in second period. For pharmacodynamic analysis, 24 hours blood pressure changes were monitored by ambulatory blood pressure monitoring. Twenty-four hour blood pressure changes were matched to valsartan concentration and analyzed by correlation analysis. Changes in blood pressure pattern were also analyzed. Subjects were divided into responder, non-responder, and reverse responder according to pre- and post- 24 hours blood monitoring results. For determination of pharmacokinetic parameters, plasma concentration of valsartan was measured by a validated ultra-performance liquid chromatography-tandem mass spectrometry method. Pharmacokinetic parameters including area under the plasma concentration versus time curve from 0 hour to the last measurable concentration (AUCt), area under the plasma concentration versus time curve extrapolated to infinity, maximum plasma concentration (Cmax), and time required to reach maximum plasma concentration (Tmax) were calculated by noncompartmental models in the BA-CALC 2008 program ver. 1.0.0.
RESULTS
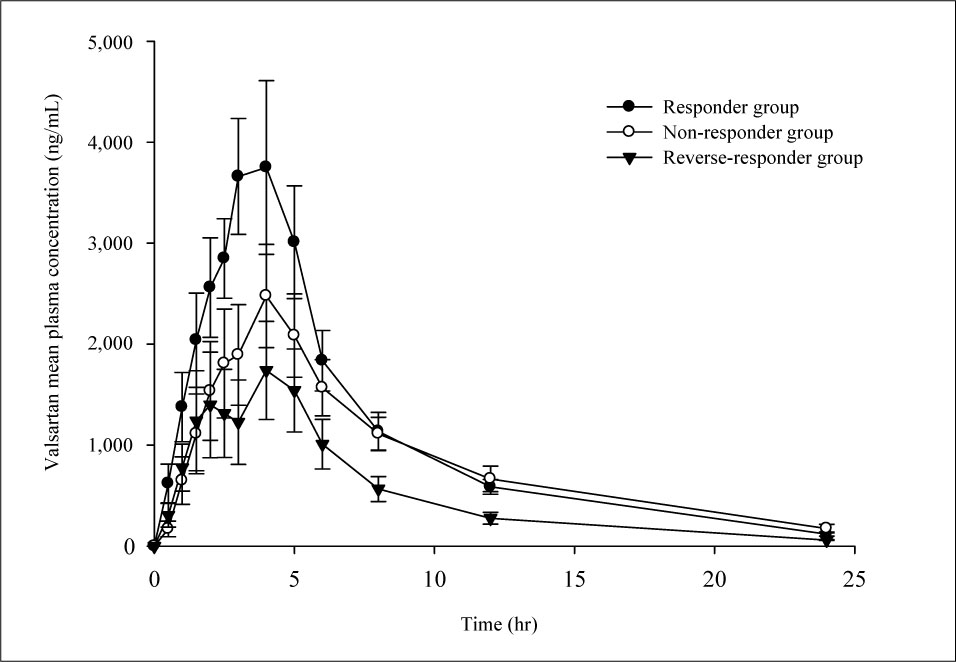
There were no significant associations between blood pressure changes and pharmacokinetic parameters of valsartan. Blood pressure pattern change analysis showed significant results. For AUCt, total amount of absorbed valsartan was 25,808 +/- 6,863.0 ng.hr/mL, 20,683 +/- 8,782.7 ng.hr/mL, and 12,502 +/- 5,566.6 ng.hr/mL in responder, non-responder, and reverse responder, respectively (p = 0.041). In C max, maximum concentration of valsartan was 4,314 +/- 1,522.6 ng/mL, 2,588 +/- 1,273.9 ng/mL, and 2,056 +/- 1,075.5 ng/mL, respectively.
CONCLUSIONS
These results showed that response to valsartan was not associated with blood concentration in healthy volunteers and changes in blood pressure patterns to valsartan might be associated with the amount of drugs which are absorbed to subjects.
MeSH Terms
Figure
Reference
-
1. Criscione L, de Gasparo M, Buhlmayer P, Whitebread S, Ramjoue HP, Wood J. Pharmacological profile of valsartan: a potent, orally active, nonpeptide antagonist of the angiotensin II AT1-receptor subtype. Br J Pharmacol. 1993. 110:761–771.
Article2. Corea L, Cardoni O, Fogari R, Innocenti P, Porcellati C, Provvidenza M, et al. Valsartan, a new angiotensin II antagonist for the treatment of essential hypertension: a comparative study of the efficacy and safety against amlodipine. Clin Pharmacol Ther. 1996. 60:341–346.
Article3. Mazayev VP, Fomina IG, Kazakov EN, Sulimov VA, Zvereva TV, Lyusov VA, et al. Valsartan in heart failure patients previously untreated with an ACE inhibitor. Int J Cardiol. 1998. 65:239–246.
Article4. Cohn JN. Improving outcomes in congestive heart failure: Val-HeFT. Valsartan in Heart Failure Trial. Cardiology. 1999. 91:Suppl 1. 19–22.5. Neutel J, Weber M, Pool J, Smith D, Fitzsimmons S, Chiang YT, et al. Valsartan, a new angiotensin II antagonist: antihypertensive effects over 24 hours. Clin Ther. 1997. 19:447–458.
Article6. Lasko BH, Laplante A, Hebert D, Bonnefis-Boyer S. Canadian valsartan study in patients with mild-to-moderate hypertension. Blood Press Monit. 2001. 6:91–99.
Article7. Shimizu M, Kario K. Perfect 24-Hour BP Management for High-Risk Hypertension. J Korean Soc Hypertens. 2010. 16:1–8.8. Korean Society of Hypertension. Blood pressure monitoring guidelines. 2007. Seoul: Korean Society of Hypertension.9. Sioufi A, Marfil F, Jaouen A, Cardot JM, Godbillon J, Ezzet F, et al. The effect of age on the pharmacokinetics of valsartan. Biopharm Drug Dispos. 1998. 19:237–244.
Article10. Okumura M, Iwai M, Ide A, Mogi M, Ito M, Horiuchi M. Sex difference in vascular injury and the vasoprotective effect of valsartan are related to differential AT2 receptor expression. Hypertension. 2005. 46:577–583.11. Asmar R, Oparil S. Comparison of the antihypertensive efficacy of irbesartan/HCTZ and valsartan/HCTZ combination therapy: impact of age and gender. Clin Exp Hypertens. 2010. 32:499–503.
Article12. Konoshita T, Kato N, Fuchs S, Mizuno S, Aoyama C, Motomura M, et al. Genetic variant of the Renin-Angiotensin system and diabetes influences blood pressure response to Angiotensin receptor blockers. Diabetes Care. 2009. 32:1485–1490.
Article13. Li H, Wang Y, Jiang Y, Tang Y, Wang J, Zhao L, et al. A liquid chromatography/tandem mass spectrometry method for the simultaneous quantification of valsartan and hydrochlorothiazide in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. 852:436–442.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A semi-compartmental model describing the pharmacokinetic-pharmacodynamic relationship
- Obesity and anesthetic pharmacology: simulation of target-controlled infusion models of propofol and remifentanil
- Target controlled infusion for total intravenous anesthesia in children
- Basic Principles of Drug Interaction
- Neuromuscular blockade and pharmacokinetic-pharmacodynamic modeling