J Periodontal Implant Sci.
2011 Apr;41(2):73-78.
Effect of seeding using an avidin-biotin binding system on the attachment of periodontal ligament fibroblasts to nanohydroxyapatite scaffolds: three-dimensional culture
- Affiliations
-
- 1Department of periodontology, Institute for Periodontal Tissue Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- 2Department of Dental Biomaterials and Bioengineering, Research Institute of Dental Biomaterials and Bioengineering, Yonsei University College of Dentistry, Seoul, Korea.
Abstract
- PURPOSE
For periodontal tissue engineering, it is a primary requisite and a challenge to select the optimum types of cells, properties of scaffold, and growth factor combination to reconstruct a specific tissue in its natural form and with the appropriate function. Owing to fundamental disadvantages associated with using a two-dimensional substrate, several methods of seeding cells into three-dimensional scaffolds have been reported and the authors have asserted its usefulness and effectiveness. In this study, we explore the cell attachment of periodontal ligament fibroblasts on nanohydroxyapatite (n-HA) scaffold using avidin biotin binding system (ABBS).
METHODS
Human periodontal ligament fibroblasts were isolated from the health tooth extracted for the purpose of orthodontic procedure. HA nanoparticles were prepared and Ca(NO3)2-4H2O and (OC2H5)3P were selected as precursors of HA sol. The final scaffold was 8 mm in diameter and 3 mm in height disk with porosity value of 81.55%. 1x10(5) periodontal ligament fibroblasts were applied to each scaffold. The cells were seeded into scaffolds by static, agitating and ABBS seeding method.
RESULTS
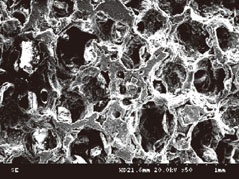
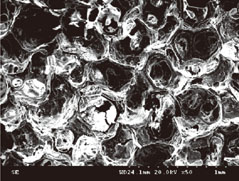
The number of periodontal ligament fibroblasts attached was greater for ABBS seeding method than for static or agitating method (P<0.05). No meaningful difference has been observed among seeding methods with scanning electron microscopy images. However, increased strength of cell attachment of ABBS could be deduced from the high affinity between avidin and biotin (Kd=10(-15) M).
CONCLUSIONS
The high-affinity ABBS enhances the ability of periodontal ligament fibroblasts to attach to three-dimensionally constructed n-HA scaffolds.
MeSH Terms
Figure
Reference
-
1. Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000. 24:253–269.
Article2. Lynch SE, Marx RE, Nevins M, Wisner-Lynch LA. Tissue engineering: applications in maxillofacial surgery and periodontics. 2008. 2nd ed. Chicago: Quintessence.3. McCulloch CA, Lekic P, McKee MD. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontol 2000. 2000. 24:56–72.
Article4. Nyman S, Gottlow J, Karring T, Lindhe J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol. 1982. 9:257–265.
Article5. Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982. 9:290–296.
Article6. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004. 364:149–155.
Article7. Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008. 14:61–86.
Article8. Zhao F, Yin Y, Lu WW, Leong JC, Zhang W, Zhang J, et al. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials. 2002. 23:3227–3234.
Article9. Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007. 28:3338–3348.
Article10. Kim SS, Sundback CA, Kaihara S, Benvenuto MS, Kim BS, Mooney DJ, et al. Dynamic seeding and in vitro culture of hepatocytes in a flow perfusion system. Tissue Eng. 2000. 6:39–44.
Article11. Yang TH, Miyoshi H, Ohshima N. Novel cell immobilization method utilizing centrifugal force to achieve high-density hepatocyte culture in porous scaffold. J Biomed Mater Res. 2001. 55:379–386.
Article12. Takahashi Y, Tabata Y. Homogeneous seeding of mesenchymal stem cells into nonwoven fabric for tissue engineering. Tissue Eng. 2003. 9:931–938.
Article13. Lueders C, Sodian R, Shakibaei M, Hetzer R. Short-term culture of human neonatal myofibroblasts seeded using a novel three-dimensional rotary seeding device. ASAIO J. 2006. 52:310–314.
Article14. Balcells M, Edelman ER. Effect of pre-adsorbed proteins on attachment, proliferation, and function of endothelial cells. J Cell Physiol. 2002. 191:155–161.
Article15. Dekker A, Poot AA, van Mourik JA, Workel MP, Beugeling T, Bantjes A, et al. Improved adhesion and proliferation of human endothelial cells on polyethylene precoated with monoclonal antibodies directed against cell membrane antigens and extracellular matrix proteins. Thromb Haemost. 1991. 66:715–724.
Article16. Tsai WB, Wang MC. Effects of an avidin-biotin binding system on chondrocyte adhesion and growth on biodegradable polymers. Macromol Biosci. 2005. 5:214–221.
Article17. Saggiowoyansky J, Scott CE, Minnear WP. Processing of porous ceramics. Am ceram soc bull. 1992. 71:1674–1682.18. Green NM. Avidin. Adv Protein Chem. 1975. 29:85–133.
Article19. Akiyama SK, Yamada KM. The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem. 1985. 260:4492–4500.
Article20. Terranova VP, Rao CN, Kalebic T, Margulies IM, Liotta LA. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983. 80:444–448.
Article21. Kojima N, Matsuo T, Sakai Y. Rapid hepatic cell attachment onto biodegradable polymer surfaces without toxicity using an avidin-biotin binding system. Biomaterials. 2006. 27:4904–4910.
Article22. Lang H, Schüler N, Nolden R. Attachment formation following replantation of cultured cells into periodontal defects--a study in minipigs. J Dent Res. 1998. 77:393–405.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Avidin and Biotin in Attachment of Human Adipose Stem Cells to Micronized Acellular Dermal Matrix
- Effect of Inorganic Polyphosphate on Cultured Periodontal Ligament Cells
- Autotransplantation using the acellular dermal matrix seeded by periodontal ligament fibroblasts in minipig: histological evaluation as potential periodontal ligament substitutes
- The 3-dimensional attachment of human periodontal ligament fibroblasts on periodontally involved root surface following treatment with EDTA: A SEM study in vitro
- The effect of rhBMP-2 on the osteoblastic differentiation of human periodontal ligament cells and gingival fibroblasts in vitro