J Periodontal Implant Sci.
2010 Aug;40(4):180-187.
Spontaneous healing capacity of rabbit cranial defects of various sizes
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
Abstract
- PURPOSE
This study evaluated the spontaneous healing capacity of surgically produced cranial defects in rabbits with different healing periods in order to determine the critical size defect (CSD) of the rabbit cranium.
METHODS
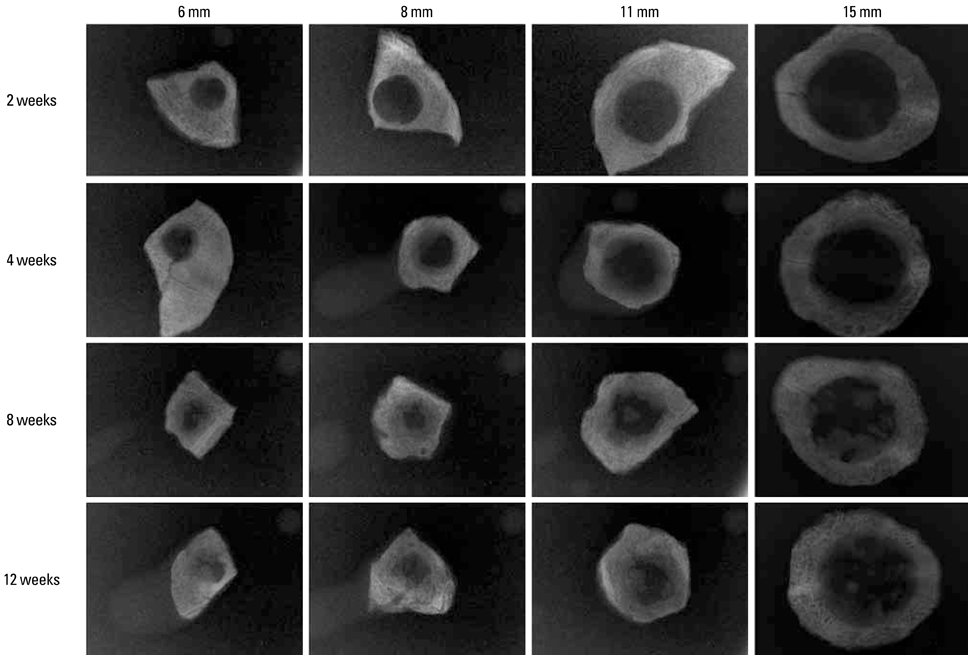
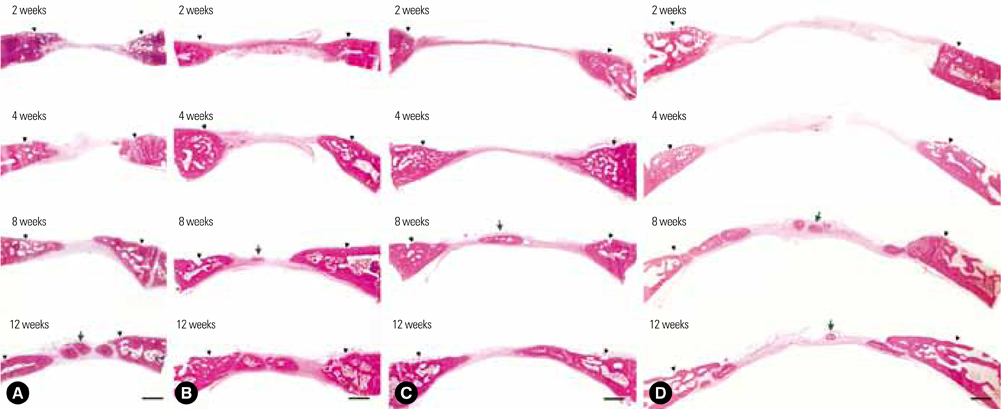
Thirty-two New Zealand white rabbits were used in this study. Defects of three sizes (6, 8, and 11 mm) were created in each of 16 randomly selected rabbits, and 15-mm defects were created individually in another 16 rabbits. The defects were analyzed using radiography, histologic analysis, and histometric analysis after the animal was sacrificed at 2, 4, 8, or 12 weeks postoperatively. Four samples were analyzed for each size of defect and each healing period.
RESULTS
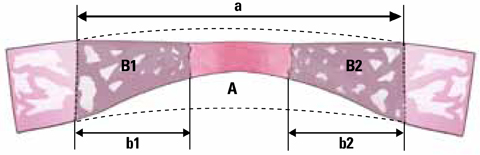
The radiographic findings indicated that defect filling gradually increased over time and that smaller defects were covered with a greater amount of radiopaque substance. Bony islands were observed at 8 weeks at the center of the defect in both histologic sections and radiographs. Histometrical values show that it was impossible to determine the precise CSD of the rabbit cranium. However, the innate healing capacity that originates from the defect margin was found to be constant regardless of the defect size.
CONCLUSIONS
The results obtained for the spontaneous healing capacity of rabbit cranial defects over time and the underlying factors may provide useful guidelines for the development of a rabbit cranial model for in vivo investigations of new bone materials.
Keyword
Figure
Reference
-
1. Bos GD, Goldberg VM, Powell AE, Heiple KG, Zika JM. The effect of histocompatibility matching on canine frozen bone allografts. J Bone Joint Surg Am. 1983. 65:89–96.
Article2. Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986. 205:299–308.
Article3. Bodde EW, Spauwen PH, Mikos AG, Jansen JA. Closing capacity of segmental radius defects in rabbits. J Biomed Mater Res A. 2008. 85:206–217.
Article4. Frame JW. A convenient animal model for testing bone substitute materials. J Oral Surg. 1980. 38:176–180.5. Le Guehennec L, Goyenvalle E, Aguado E, Houchmand-Cuny M, Enkel B, Pilet P, et al. Small-animal models for testing macroporous ceramic bone substitutes. J Biomed Mater Res B Appl Biomater. 2005. 72:69–78.
Article6. Gilsanz V, Roe TF, Gibbens DT, Schulz EE, Carlson ME, Gonzalez O, et al. Effect of sex steroids on peak bone density of growing rabbits. Am J Physiol. 1988. 255:E416–E421.
Article7. Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone. 1995. 16:277S–284S.
Article8. Castaneda S, Largo R, Calvo E, Rodriguez-Salvanes F, Marcos ME, Diaz-Curiel M, et al. Bone mineral measurements of subchondral and trabecular bone in healthy and osteoporotic rabbits. Skeletal Radiol. 2006. 35:34–41.
Article9. Pripatnanont P, Nuntanaranont T, Vongvatcharanon S. Proportion of deproteinized bovine bone and autogenous bone affects bone formation in the treatment of calvarial defects in rabbits. Int J Oral Maxillofac Surg. 2009. 38:356–362.
Article10. Gosain AK, Santoro TD, Song LS, Capel CC, Sudhakar PV, Matloub HS. Osteogenesis in calvarial defects: contribution of the dura, the pericranium, and the surrounding bone in adult versus infant animals. Plast Reconstr Surg. 2003. 112:515–527.
Article11. Xu S, Lin K, Wang Z, Chang J, Wang L, Lu J, et al. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials. 2008. 29:2588–2596.
Article12. Hammerle CH, Schmid J, Olah AJ, Lang NP. Osseous healing of experimentally created defects in the calvaria of rabbits using guided bone regeneration. A pilot study. Clin Oral Implants Res. 1992. 3:144–147.13. Kramer IR, Killey HC, Wright HC. A histological and radiological comparison of the healing of defects in the rabbit calvarium with and without implanted heterogeneous anorganic bone. Arch Oral Biol. 1968. 13:1095–1106.
Article14. Lundgren D, Nyman S, Mathisen T, Isaksson S, Klinge B. Guided bone regeneration of cranial defects, using biodegradable barriers: an experimental pilot study in the rabbit. J Craniomaxillofac Surg. 1992. 20:257–260.
Article15. Pallesen L, Schou S, Aaboe M, Hjorting-Hansen E, Nattestad A, Melsen F. Influence of particle size of autogenous bone grafts on the early stages of bone regeneration: a histologic and stereologic study in rabbit calvarium. Int J Oral Maxillofac Implants. 2002. 17:498–506.16. Shand JM, Heggie AA, Holmes AD, Holmes W. Allogeneic bone grafting of calvarial defects: an experimental study in the rabbit. Int J Oral Maxillofac Surg. 2002. 31:525–531.
Article17. Cameron S. Hand-held computers in medicine. Can Fam Physician. 2002. 48:111–112.18. Nagata MJ, Melo LG, Messora MR, Bomfim SR, Fucini SE, Garcia VG, et al. Effect of platelet-rich plasma on bone healing of autogenous bone grafts in critical-size defects. J Clin Periodontol. 2009. 36:775–783.
Article19. Melo LG, Nagata MJ, Bosco AF, Ribeiro LL, Leite CM. Bone healing in surgically created defects treated with either bioactive glass particles, a calcium sulfate barrier, or a combination of both materials. A histological and histometric study in rat tibias. Clin Oral Implants Res. 2005. 16:683–691.
Article20. Cavalcanti SC, Pereira CL, Mazzonetto R, de Moraes M, Moreira RW. Histological and histomorphometric analyses of calcium phosphate cement in rabbit calvaria. J Craniomaxillofac Surg. 2008. 36:354–359.
Article21. Durmus E, Celik I, Aydin MF, Yildirim G, Sur E. Evaluation of the biocompatibility and osteoproductive activity of ostrich eggshell powder in experimentally induced calvarial defects in rabbits. J Biomed Mater Res B Appl Biomater. 2008. 86:82–89.22. Torres J, Tamimi FM, Tresguerres IF, Alkhraisat MH, Khraisat A, Lopez-Cabarcos E, et al. Effect of solely applied platelet-rich plasma on osseous regeneration compared to Bio-Oss: a morphometric and densitometric study on rabbit calvaria. Clin Implant Dent Relat Res. 2008. 10:106–112.
Article23. Glowacki J, Altobelli D, Mulliken JB. Fate of mineralized and demineralized osseous implants in cranial defects. Calcif Tissue Int. 1981. 33:71–76.
Article24. Strates BS, Connolly JF. Osteogenesis in cranial defects and diffusion chambers. Comparison in rabbits of bone matrix, marrow, and collagen implants. Acta Orthop Scand. 1989. 60:200–203.
Article25. Urist MR, Nilsson O, Rasmussen J, Hirota W, Lovell T, Schmalzreid T, et al. Bone regeneration under the influence of a bone morphogenetic protein (BMP) beta tricalcium phosphate (TCP) composite in skull trephine defects in dogs. Clin Orthop Relat Res. 1987. 214:295–304.
Article26. Vogeler KT, Redenz E, Walter H, Martin G. Deutsche Gesellschaft fur Chirurgie. Bernhard Heines Versuche uber Knochenregeneration: sein Leben und seine Zeit. 1926. Berlin: J. Springer.27. Greenwald JA, Mehrara BJ, Spector JA, Chin GS, Steinbrech DS, Saadeh PB, et al. Biomolecular mechanisms of calvarial bone induction: immature versus mature dura mater. Plast Reconstr Surg. 2000. 105:1382–1392.
Article28. Huh JY, Choi BH, Kim BY, Lee SH, Zhu SJ, Jung JH. Critical size defect in the canine mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005. 100:296–301.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histomorphometric evaluation of bone healing capacity of epigallocatechin-3-gallate-loaded β-TCP bone substitute in rabbit calvarial defects
- Spontaneous Bone Regeneration in Surgically Induced Bone Defects in Young Rabbits
- The experimental study of the bone regeneration on beta-TCP in rabbit cranial bone
- Comparable bone healing capacity of different bone graft matrices in a rabbit segmental defect model
- A comparative study of various type of autogeneous bone graft on the rabbit-skull defect healing