Nat Prod Sci.
2016 Jun;22(2):122-128. 10.20307/nps.2016.22.2.122.
Cytotoxic, Anti-Inflammatory and Adipogenic Effects of Inophyllum D, Calanone, Isocordato-oblongic acid, and Morelloflavone on Cell Lines
- Affiliations
-
- 1Department of Pharmaceutical Technology, Faculty of Pharmacy, International Islamic University Malaysia, Jalan Sultan Ahmad Shah, Bandar Indera Mahkota, 25200 Pahang, Malaysia. mtaher@iium.edu.my
- 2Department of Biomedical Science, Faculty of Science, International Islamic University Malaysia, Jalan Sultan Ahmad Shah, Bandar Indera Mahkota, 25200 Pahang, Malaysia.
- 3Department of Chemistry, Faculty of Science, International Islamic University Malaysia, Jalan Sultan Ahmad Shah, Bandar Indera Mahkota, 25200 Pahang, Malaysia.
- 4Department of Chemistry, Faculty of Science, Universiti Teknologi Malaysia, 81310 Johor, Malaysia.
- KMID: 2328878
- DOI: http://doi.org/10.20307/nps.2016.22.2.122
Abstract
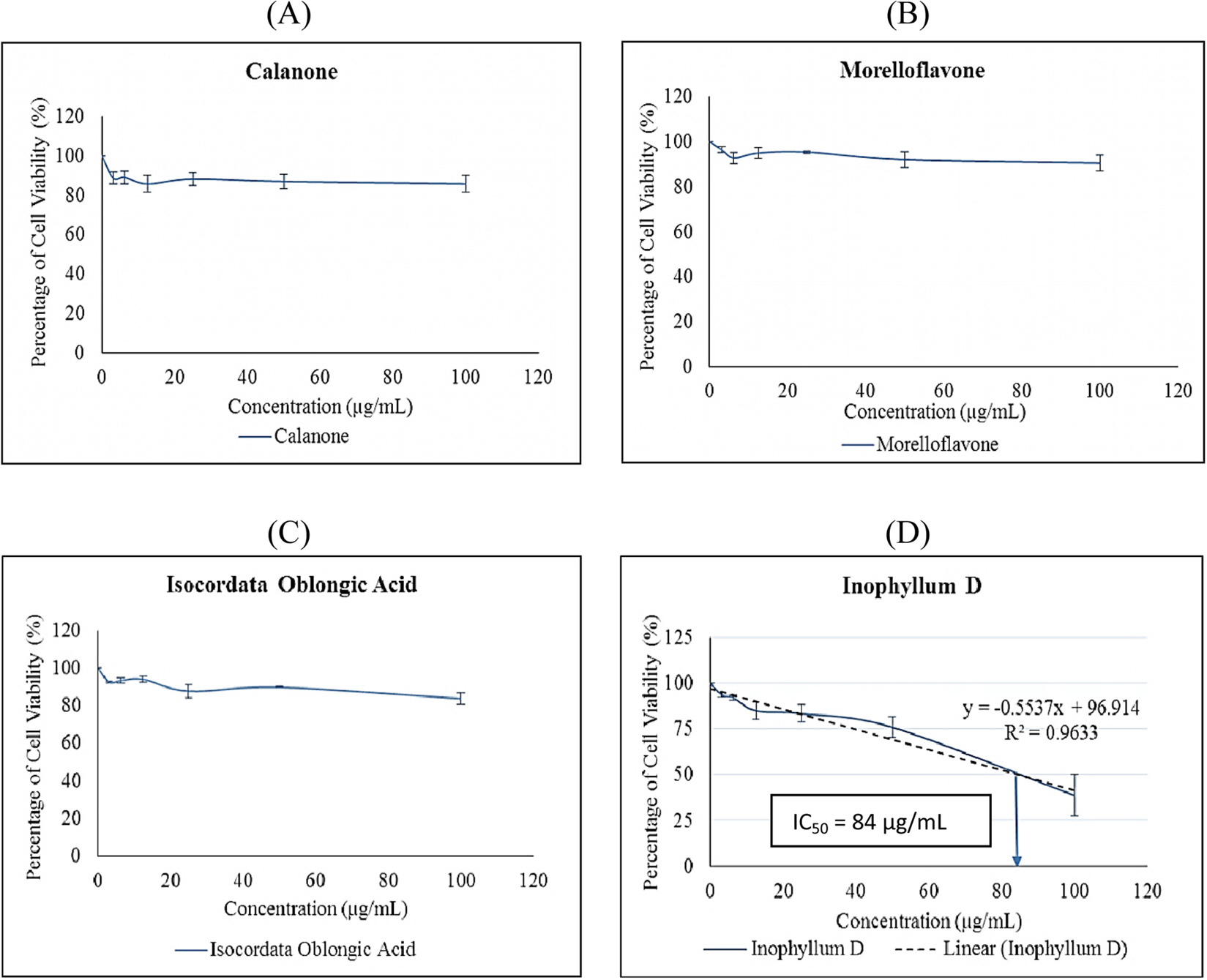
- This paper reports in vitro cytotoxic, anti-inflammatory and adipocyte diffentiation with adipogenic effects of coumarins inophyllum D (1) and calanone (2), and a chromanone carboxylic acid namely isocordato-oblongic acid (3) isolated from Calophyllum symingtonianum as well as a biflavonoid morelloflavon e (4) isolated from Garcinia prainiana on MCF-7 breast adenocarcinoma RAW 264.7 macrophages and 3T3-L1 preadipocytes cells, respectively. The cytotoxicity study on MCF-7 cell was conducted by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Meanwhile, the study of anti-inflammatory effects in RAW 264.7 macrophages and adipogenic effects on 3T3-L1 pre-adipocytes were conducted through nitrite determination assay and induction of adipocyte differentiation, respectively. In the cytotoxicity study, inophyllum D (1) was the only compounds that exhibited significant cytotoxic effect against MCF-7 cell with ICâ‚…â‚€ of 84 µg/mL. Further, all by inhibiting the compounds have shown anti-inflammatory effects in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages of nitrite concentration with production. In addition, the compounds also exhibited adipogenic effects on 3T3-L1 pre-adipocytes by stimulating lipid formation. Thus, this study may provide significant input in discovery of the potential effects cytotoxic, anti-inflammatory and adipogenic agents.
Keyword
MeSH Terms
Figure
Reference
-
(1). Hanson J. R.Royal Society of Chemistry. 2003; 17.(2). Croteau R., Kutchan T.M.; Lewis, N.G. Biochem. Mol. Biol. Plants. 2000; 24:1250–1319.(3). Brahmachari G.Bioactive Natural Products; John Wiley & Sons: United Kingdom. 2015; 1–199.(4). Aminudin N. I., Ahmad F., Taher M., Zulkifli R. M.Nat. Prod. Commun. 2015; 10:1585–1587.(5). Waterman P. G., Crichton E. G.Phytochemistry. 1980; 19:2723–2726.(6). Mosmann T. J.Immunol. Methods. 1983; 65:55–63.(7). Lee H. S., Ryu D. S., Lee G. S., Lee D. S. J.Ethnopharmacol. 2012; 140:271–276.(8). Susanti D., Amiroudine M. Z., Rezali M. F., Taher M.Nat. Prod. Res. 2013; 27:417–424.(9). Itoigawa M., Ito C., Tan H. T., Kuchide M., Tokuda H., Nishino H., Furukawa H.Cancer Lett. 2001; 169:15–19.(10). Osman A. M., Bayoumi H. M., Al-Harthi S. E., Damanhouri Z. A., ElShal M. F.Cancer Cell Int. 2012; 12:47–53.(11). Gao Y., Jiang W., Dong C., Li C., Fu X., Min L., Tian J., Jin H., Shen J.Toxicol. In Vitro. 2012; 26(1):1–6.(12). Nahar P. P., Driscoll M. V., Li L., Slitt A. L., Seeram N. P. J.Funct. Foods. 2014; 6:126–136.(13). Kumar V., Abbas A. K., Fausto N., Mitchell R.Robbins Basic Pathology: 8th Edition; Saunders/Elsevier: Philadelphia. 2007; 9.(14). Kim H. K., Cheon B. S., Kim Y. H., Kim S. Y., Kim H. P.Biochem. Pharmacol. 1999; 58:759–765.(15). Macia L., Viltart O., Verwaerde C., Delacre M., Delanoye A., Grangette C., Wolowczuk I.Genes Nutr. 2006; 1:189–212.(16). Yun J. W.Phytochemistry. 2010; 71:1625–1641.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte
- A Cytotoxic Lupeol Fatty Acid Ester and Other Pentacyclic Triterpenes from Salvadora persica Seeds
- In Vitro Potentiation of 5-Fluorouracii-lnduced Cytotoxicity by Leucovorin in Human Bladder Cancer Cell Lines
- Tolfenamic Acid Inhibits the Proliferation, Migration, and Invasion of Nasopharyngeal Carcinoma: Involvement of p38-Mediated Down-Regulation of Slug
- Ellagic Acid Shows Different Anti-proliferative Effects Between the MDA-MB-231 and MCF-7 Human Breast Cancer Cell Lines