Ann Rehabil Med.
2016 Jun;40(3):420-431. 10.5535/arm.2016.40.3.420.
Virtual Reality-Guided Motor Imagery Increases Corticomotor Excitability in Healthy Volunteers and Stroke Patients
- Affiliations
-
- 1Department of Rehabilitation Medicine, Eulji Hospital, Eulji University School of Medicine, Seoul, Korea. md52516@hanmail.net
- 2Department of Biomedical Engineering, Keimyung University, Daegu, Korea.
- KMID: 2327603
- DOI: http://doi.org/10.5535/arm.2016.40.3.420
Abstract
OBJECTIVE
To investigate the effects of using motor imagery (MI) in combination with a virtual reality (VR) program on healthy volunteers and stroke patients. In addition, this study investigated whether task variability within the VR-guided MI programs would influence corticomotor excitability.
METHODS
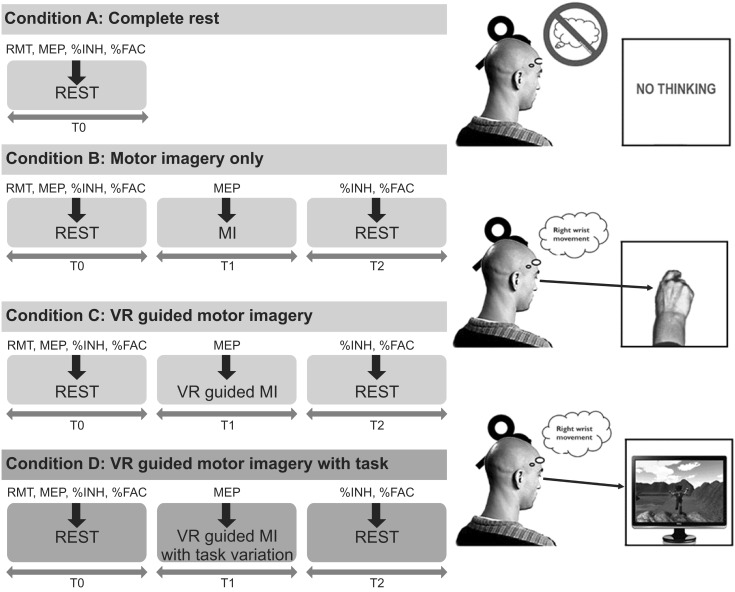
The present study included 15 stroke patients and 15 healthy right-handed volunteers who were presented with four different conditions in a random order: rest, MI alone, VR-guided MI, and VR-guided MI with task variability. The corticomotor excitability of each participant was assessed before, during, and after each condition by measuring changes in the various parameters of motor-evoked potentials (MEPs) of the extensor carpi radials (ECR). Changes in intracortical inhibition (ICI) and intracortical facilitation (ICF) were calculated after each condition as percentages of inhibition (%INH) and facilitation (%FAC) at rest.
RESULTS
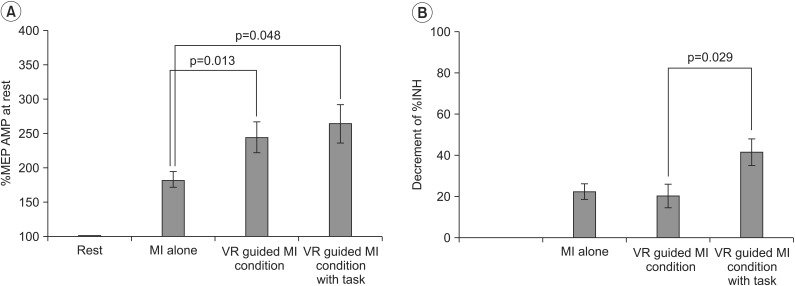
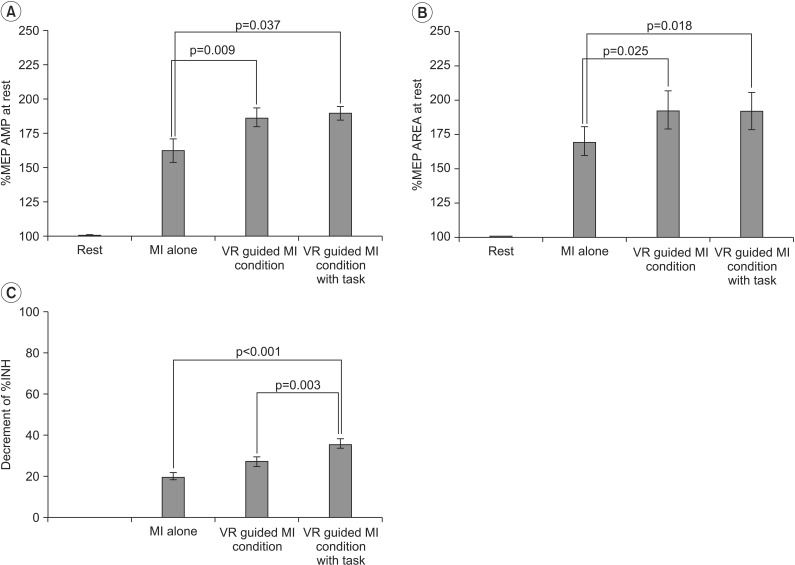
In both groups, the increases in MEP amplitudes were greater during the two VR-guided MI conditions than during MI alone. Additionally, the reductions in ECR %INH in both groups were greater under the condition involving VR-guided MI with task variability than under that involving VR-guided MI with regular interval.
CONCLUSION
The corticomotor excitability elicited by MI using a VR avatar representation was greater than that elicited by MI with real body observations. Furthermore, the use of task variability in a VR program may enhance neural regeneration after stroke by reducing ICI. The present findings support the use of various VR programs as well as the concept of combining MI with VR programs for neurorehabilitation.
Keyword
MeSH Terms
Figure
Reference
-
1. Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005; 352:1677–1684. PMID: 15843670.2. Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev. 2014; 11:CD010820. PMID: 25387001.
Article3. Mulder T. Motor imagery and action observation: cognitive tools for rehabilitation. J Neural Transm (Vienna). 2007; 114:1265–1278. PMID: 17579805.
Article4. Lotze M, Montoya P, Erb M, Hulsmann E, Flor H, Klose U, et al. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci. 1999; 11:491–501. PMID: 10511638.
Article5. Kho AY, Liu KP, Chung RC. Meta-analysis on the effect of mental imagery on motor recovery of the hemiplegic upper extremity function. Aust Occup Ther J. 2014; 61:38–48. PMID: 24138081.
Article6. Subramanian SK, Lourenco CB, Chilingaryan G, Sveistrup H, Levin MF. Arm motor recovery using a virtual reality intervention in chronic stroke: randomized control trial. Neurorehabil Neural Repair. 2013; 27:13–23. PMID: 22785001.7. Turolla A, Dam M, Ventura L, Tonin P, Agostini M, Zucconi C, et al. Virtual reality for the rehabilitation of the upper limb motor function after stroke: a prospective controlled trial. J Neuroeng Rehabil. 2013; 10:85. PMID: 23914733.
Article8. Prochnow D, Bermudez i Badia S, Schmidt J, Duff A, Brunheim S, Kleiser R, et al. A functional magnetic resonance imaging study of visuomotor processing in a virtual reality-based paradigm: rehabilitation gaming system. Eur J Neurosci. 2013; 37:1441–1447. PMID: 23414211.
Article9. Diers M, Kamping S, Kirsch P, Rance M, Bekrater-Bodmann R, Foell J, et al. Illusion-related brain activations: a new virtual reality mirror box system for use during functional magnetic resonance imaging. Brain Res. 2015; 1594:173–182. PMID: 25446453.
Article10. Brunner I, Skouen JS, Hofstad H, Strand LI, Becker F, Sanders AM, et al. Virtual reality training for upper extremity in subacute stroke (VIRTUES): study protocol for a randomized controlled multicenter trial. BMC Neurol. 2014; 14:186. PMID: 25261187.
Article11. Fluet GG, Deutsch JE. Virtual reality for sensorimotor rehabilitation post-stroke: the promise and current state of the field. Curr Phys Med Rehabil Rep. 2013; 1:9–20. PMID: 24579058.
Article12. Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke. 2003; 34:2653–2658. PMID: 14551397.13. Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000; 111:671–676. PMID: 10727918.
Article14. Leonard G, Tremblay F. Corticomotor facilitation associated with observation, imagery and imitation of hand actions: a comparative study in young and old adults. Exp Brain Res. 2007; 177:167–175. PMID: 16947064.
Article15. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971; 9:97–113. PMID: 5146491.
Article16. Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009; 120:2008–2039. PMID: 19833552.
Article17. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12:189–198. PMID: 1202204.18. Roberts R, Callow N, Hardy L, Markland D, Bringer J. Movement imagery ability: development and assessment of a revised version of the vividness of movement imagery questionnaire. J Sport Exerc Psychol. 2008; 30:200–221. PMID: 18490791.
Article19. Hashimoto R, Rothwell JC. Dynamic changes in corticospinal excitability during motor imagery. Exp Brain Res. 1999; 125:75–81. PMID: 10100979.
Article20. Naish KR, Houston-Price C, Bremner AJ, Holmes NP. Effects of action observation on corticospinal excitability: muscle specificity, direction, and timing of the mirror response. Neuropsychologia. 2014; 64:331–348. PMID: 25281883.
Article21. Stinear CM, Byblow WD, Steyvers M, Levin O, Swinnen SP. Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp Brain Res. 2006; 168:157–164. PMID: 16078024.
Article22. Buccino G, Solodkin A, Small SL. Functions of the mirror neuron system: implications for neurorehabilitation. Cogn Behav Neurol. 2006; 19:55–63. PMID: 16633020.
Article23. Sharma N, Baron JC, Rowe JB. Motor imagery after stroke: relating outcome to motor network connectivity. Ann Neurol. 2009; 66:604–616. PMID: 19938103.
Article24. Kang YJ, Ku J, Kim HJ, Park HK. Facilitation of corticospinal excitability according to motor imagery and mirror therapy in healthy subjects and stroke patients. Ann Rehabil Med. 2011; 35:747–758. PMID: 22506202.
Article25. Rossini PM, Rossi S, Pasqualetti P, Tecchio F. Cortico spinal excitability modulation to hand muscles during movement imagery. Cereb Cortex. 1999; 9:161–167. PMID: 10220228.26. Tai YF, Scherfler C, Brooks DJ, Sawamoto N, Castiello U. The human premotor cortex is 'mirror' only for biological actions. Curr Biol. 2004; 14:117–120. PMID: 14738732.
Article27. Gazzola V, Rizzolatti G, Wicker B, Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage. 2007; 35:1674–1684. PMID: 17395490.
Article28. Kang YJ, Park HK, Kim HJ, Lim T, Ku J, Cho S, et al. Upper extremity rehabilitation of stroke: facilitation of corticospinal excitability using virtual mirror paradigm. J Neuroeng Rehabil. 2012; 9:71. PMID: 23035951.
Article29. Aziz-Zadeh L, Iacoboni M, Zaidel E, Wilson S, Mazziotta J. Left hemisphere motor facilitation in response to manual action sounds. Eur J Neurosci. 2004; 19:2609–2612. PMID: 15128415.
Article30. Ljubisavljevic M. Transcranial magnetic stimulation and the motor learning-associated cortical plasticity. Exp Brain Res. 2006; 173:215–222. PMID: 16733699.
Article31. Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991; 251:944–947. PMID: 2000496.
Article32. Avanzino L, Gueugneau N, Bisio A, Ruggeri P, Papaxanthis C, Bove M. Motor cortical plasticity induced by motor learning through mental practice. Front Behav Neurosci. 2015; 9:105. PMID: 25972791.
Article33. Stinear CM, Byblow WD. Modulation of corticospinal excitability and intracortical inhibition during motor imagery is task-dependent. Exp Brain Res. 2004; 157:351–358. PMID: 14997259.
Article34. Mirelman A, Maidan I, Deutsch JE. Virtual reality and motor imagery: promising tools for assessment and therapy in Parkinson's disease. Mov Disord. 2013; 28:1597–1608. PMID: 24132848.
Article35. Roosink M, Zijdewind I. Corticospinal excitability during observation and imagery of simple and complex hand tasks: implications for motor rehabilitation. Behav Brain Res. 2010; 213:35–41. PMID: 20433871.
Article36. Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Exp Brain Res. 1998; 118:421–426. PMID: 9497149.
Article37. Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006; 19:84–90. PMID: 16415682.
Article38. Ang KK, Guan C, Chua KS, Ang BT, Kuah CW, Wang C, et al. A large clinical study on the ability of stroke patients to use an EEG-based motor imagery brain-computer interface. Clin EEG Neurosci. 2011; 42:253–258. PMID: 22208123.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Facilitation of Corticospinal Excitability According to Motor Imagery and Mirror Therapy in Healthy Subjects and Stroke Patients
- Effect of Motor Imagery on the F-Wave Parameters in Hemiparetic Stroke Survivors
- Motor Learning by Novel Therapeutic Approaches: Virtual Reality and Robotics
- Motor Imagery and Action Observation
- Assessment of Post-Stroke Cognitive Dysfunction Using 3-Dimensional Virtual Reality Program