Tuberc Respir Dis.
2016 Jul;79(3):188-192. 10.4046/trd.2016.79.3.188.
Acute Respiratory Distress Syndrome as the Initial Clinical Manifestation of an Antisynthetase Syndrome
- Affiliations
-
- 1Department of Internal Medicine, Inje University Seoul Paik Hospital, Seoul, Korea. eanee@hanmail.net
- KMID: 2326688
- DOI: http://doi.org/10.4046/trd.2016.79.3.188
Abstract
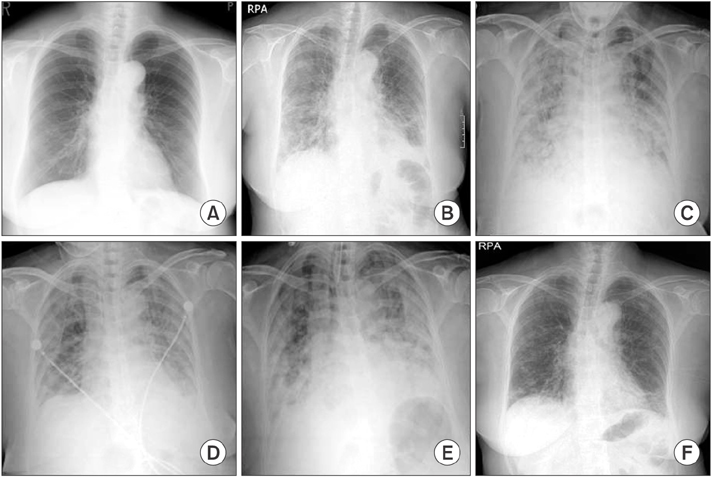
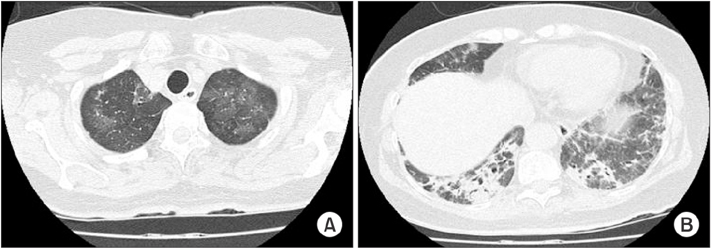
- Antisynthetase syndrome has been recognized as an important cause of autoimmune inflammatory myopathy in a subset of patients with polymyositis and dermatomyositis. It is associated with serum antibody to aminoacyl-transfer RNA synthetases and is characterized by a constellation of manifestations, including fever, myositis, interstitial lung disease, mechanic's hand-like cutaneous involvement, Raynaud phenomenon, and polyarthritis. Lung disease is the presenting feature in 50% of the cases. We report a case of a 60-year-old female with acute respiratory distress syndrome (ARDS), which later proved to be an unexpected and initial manifestation of anti-Jo-1 antibody-positive antisynthetase syndrome. The present case showed resolution of ARDS after treatment with high-dose corticosteroids. Given that steroids are not greatly beneficial in the treatment of ARDS, it is likely that the improvement of the respiratory symptoms in this patient also resulted from the prompt suppression of the inflammatory systemic response by corticosteroids.
MeSH Terms
Figure
Reference
-
1. Fathi M, Vikgren J, Boijsen M, Tylen U, Jorfeldt L, Tornling G, et al. Interstitial lung disease in polymyositis and dermatomyositis: longitudinal evaluation by pulmonary function and radiology. Arthritis Rheum. 2008; 59:677–685.2. Fathi M, Dastmalchi M, Rasmussen E, Lundberg IE, Tornling G. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004; 63:297–301.3. Saketkoo LA, Ascherman DP, Cottin V, Christopher-Stine L, Danoff SK, Oddis CV. Interstitial lung disease in idiopathic inflammatory myopathy. Curr Rheumatol Rev. 2010; 6:108–119.4. Targoff IN. Immune manifestations of inflammatory muscle disease. Rheum Dis Clin North Am. 1994; 20:857–880.5. Friedman AW, Targoff IN, Arnett FC. Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum. 1996; 26:459–467.6. Tillie-Leblond I, Wislez M, Valeyre D, Crestani B, Rabbat A, Israel-Biet D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax. 2008; 63:53–59.7. Koreeda Y, Higashimoto I, Yamamoto M, Takahashi M, Kaji K, Fujimoto M, et al. Clinical and pathological findings of interstitial lung disease patients with anti-aminoacyl-tRNA synthetase autoantibodies. Intern Med. 2010; 49:361–369.8. Bajocchi G, Piro R, Lombardini C, Cavazza A, Facciolongo N. Acute respiratory distress syndrome: an undercover antisynthetase syndrome: a case report and a review of the literature. Clin Exp Rheumatol. 2012; 30:424–428.9. Polosa R, Di Mauro C, Spampinato B, Castelli L, D'Amico G, Edwards CJ, et al. A patient with antihistidyl-tRNA synthetase positive polymyositis presenting as acute respiratory distress syndrome. J Clin Rheumatol. 2008; 14:219–221.10. Richards TJ, Eggebeen A, Gibson K, Yousem S, Fuhrman C, Gochuico BR, et al. Characterization and peripheral blood biomarker assessment of anti-Jo-1 antibody-positive interstitial lung disease. Arthritis Rheum. 2009; 60:2183–2192.11. Marie I, Hachulla E, Cherin P, Dominique S, Hatron PY, Hellot MF, et al. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum. 2002; 47:614–622.12. Huh JW, Kim DS, Lee CK, Yoo B, Seo JB, Kitaichi M, et al. Two distinct clinical types of interstitial lung disease associated with polymyositis-dermatomyositis. Respir Med. 2007; 101:1761–1769.13. Yousem SA, Gibson K, Kaminski N, Oddis CV, Ascherman DP. The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome. Mod Pathol. 2010; 23:874–880.14. Lee CS, Chen TL, Tzen CY, Lin FJ, Peng MJ, Wu CL, et al. Idiopathic inflammatory myopathy with diffuse alveolar damage. Clin Rheumatol. 2002; 21:391–396.15. Guglielmi S, Merz TM, Gugger M, Suter C, Nicod LP. Acute respiratory distress syndrome secondary to antisynthetase syndrome is reversible with tacrolimus. Eur Respir J. 2008; 31:213–217.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Dermatomyositis with Acute Interstitial Pneumonitis Manifested as Antisynthetase Syndrome
- Treatment of Acute Respiratory Distress Syndrome
- Clinical Manifestations and Diagnosis of Acute Respiratory Distress Syndrome
- Overlap Syndrome of Antisynthetase Syndrome and Rheumatoid Arthritis: A Case Report
- Acute Respiratory Distress Syndrome after Viscum album Pleurodesis for Primary Spontaneous Pneumothorax