J Korean Surg Soc.
2013 Nov;85(5):230-235.
Evaluation of cytolytic activity and phenotypic changes of circulating blood immune cells in patients with colorectal cancer by a simple preparation of peripheral blood mononuclear cells
- Affiliations
-
- 1Immunology Laboratory, Seniors Inje Hospital, Holon Center, Inje, Korea. gskjc@hanmail.net
- 2Department of Surgery, Seoul Song Do Colorectal Hospital, Seoul, Korea.
Abstract
- PURPOSE
This study aimed to assess the cytolytic activity and the phenotype of circulating blood immune cells in cancer patients by using a simple preparation of peripheral blood mononuclear cells (PBMCs).
METHODS
Peripheral blood was obtained from 94 diagnosed colorectal cancer (CRC) patients and 112 healthy donors. PBMCs were cocultured with K562 cells for 2 hours and lactate dehydrogenase released from the dead K562 cells was measured by using a spectrophotometer. Meanwhile, PBMCs were stained with fluorescence conjugated monoclonal antibodies (mAbs) and analyzed by flow cytometry.
RESULTS
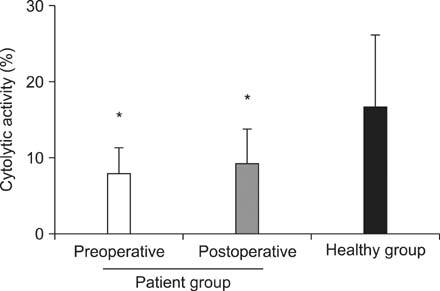
The cytolytic activity of PBMCs were significantly different between CRC patient and healthy groups (8.82% +/- 3.84% vs. 17.51% +/- 8.57%; P < 0.001). However, no significant difference in the cytolytic activity was observed after surgery in the CRC patient group (before surgery, 8.82% +/- 3.84% vs. after surgery, 9.95% +/- 4.94%; P = 0.326). In addition, the percentage of peripheral blood natural killer cells was significantly higher in the preoperative patient group than in the healthy group (19.97% +/- 11.51% vs. 15.60% +/- 5.77%, P = 0.041). In contrast, the percentage of peripheral blood lymphocytes was lower in the preoperative patient group than in the healthy group (28.41% +/- 8.31% vs. 36.4% +/- 8.6%, P < 0.001).
CONCLUSION
These results demonstrate that circulating blood immune cells of CRC patients are functionally impaired and undergo an immunophenotypic perturbation, and show that a simple preparation of PBMCs can be useful to evaluate cellular immunity in cancer.
MeSH Terms
Figure
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.2. Wu AH, Paganini-Hill A, Ross RK, Henderson BE. Alcohol, physical activity and other risk factors for colorectal cancer: a prospective study. Br J Cancer. 1987; 55:687–694.3. Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010; 138:2029–2043.e10.4. Cao Y, DePinho RA, Ernst M, Vousden K. Cancer research: past, present and future. Nat Rev Cancer. 2011; 11:749–754.5. Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001; 411:380–384.6. Smith CL, Dulphy N, Salio M, Cerundolo V. Immunotherapy of colorectal cancer. Br Med Bull. 2002; 64:181–200.7. de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006; 6:24–37.8. Sirisinha S. Insight into the mechanisms regulating immune homeostasis in health and disease. Asian Pac J Allergy Immunol. 2011; 29:1–14.9. Hourigan CS, Levitsky HI. Evaluation of current cancer immunotherapy: hemato-oncology. Cancer J. 2011; 17:309–324.10. Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002; 2:850–861.11. Waldhauer I, Steinle A. NK cells and cancer immu nosurveillance. Oncogene. 2008; 27:5932–5943.12. Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011; 2011:676198.13. Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982; 155:1823–1841.14. Miyawaki T, Taga K, Nagaoki T, Seki H, Suzuki Y, Taniguchi N. Circadian changes of T lymphocyte subsets in human peripheral blood. Clin Exp Immunol. 1984; 55:618–622.15. Ritchie AW, Oswald I, Micklem HS, Boyd JE, Elton RA, Jazwinska E, et al. Circadian variation of lymphocyte subpopulations: a study with monoclonal antibodies. Br Med J (Clin Res Ed). 1983; 286:1773–1775.16. Lin CC, Kuo YC, Huang WC, Lin CY. Natural killer cell activity in lung cancer patients. Chest. 1987; 92:1022–1024.17. Piroozmand A, Hassan ZM. Evaluation of natural killer cell activity in pre and post treated breast cancer patients. J Cancer Res Ther. 2010; 6:478–481.18. Tartter PI, Steinberg B, Barron DM, Martinelli G. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch Surg. 1987; 122:1264–1268.19. Barnett D, Walker B, Landay A, Denny TN. CD4 immunophenotyping in HIV infection. Nat Rev Microbiol. 2008; 6:11 Suppl. S7–S15.20. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006; 439:682–687.21. Biron CA, Turgiss LR, Welsh RM. Increase in NK cell number and turnover rate during acute viral infection. J Immunol. 1983; 131:1539–1545.22. Ogawa K, Hirai M, Katsube T, Murayama M, Hamaguchi K, Shimakawa T, et al. Suppression of cellular immunity by surgical stress. Surgery. 2000; 127:329–336.23. Espi A, Arenas J, Garcia-Granero E, Marti E, Lledo S. Relationship of curative surgery on natural killer cell activity in colorectal cancer. Dis Colon Rectum. 1996; 39:429–434.24. Pollock RE, Lotzova E, Stanford SD. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch Surg. 1991; 126:338–342.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of natural killer cell number and cytolytic activity during first trimester of pregnancy in recurrent spontaneous abortion patients and fertile control
- Role of Liquid Biopsies in Colorectal Cancer
- Clinical Utility of Portal Venous Circulating Tumor Cells in Pancreatic Cancer
- Detection of Circulating Tumor Cells Using Three-dimensional and Conditionally Reprogrammed Culture Methods
- Circulating Lymphoma Cells in the Peripheral Blood from 4 Cases of Mantle and T Cell Types of Non-Hodgkin's Lymphoma: Light and Electron Microscopic Morphology