J Korean Soc Spine Surg.
2002 Jun;9(2):91-97.
Scoliosis in Children after Surgery for Congenital Heart Disease
- Affiliations
-
- 1Department of Orthopedic Surgery, Seoul National University Medical College, Seoul, Korea. Choonki@plaza.snu.ac.kr
Abstract
-
STUDY DESIGN: A retrospective study.
OBJECTIVES
To analyze natural history and clinical features of scoliosis developed after surgery for congenital heart disease and to investigate the influence of cardiac surgery on the onset and progression of scoliosis.
MATERIALS AND METHODS
Three hundred and five patients who had been operated for congenital heart disease from Jan.1988 to Dec.1990 and followed up for more than 5 years were analyzed. The curve which was more than 10 degrees on radiography was defined as significant scoliosis and the patients with congenital spinal anomalies were excluded. We compared age at surgery, cardiac disease and effect of cyanosis between scoliosis and non-scoliosis group. Furthermore, in scoliosis group, prevalence, onset of significant scoliosis, and manifestation of curve according to side of thoracotomy were assessed.
RESULTS
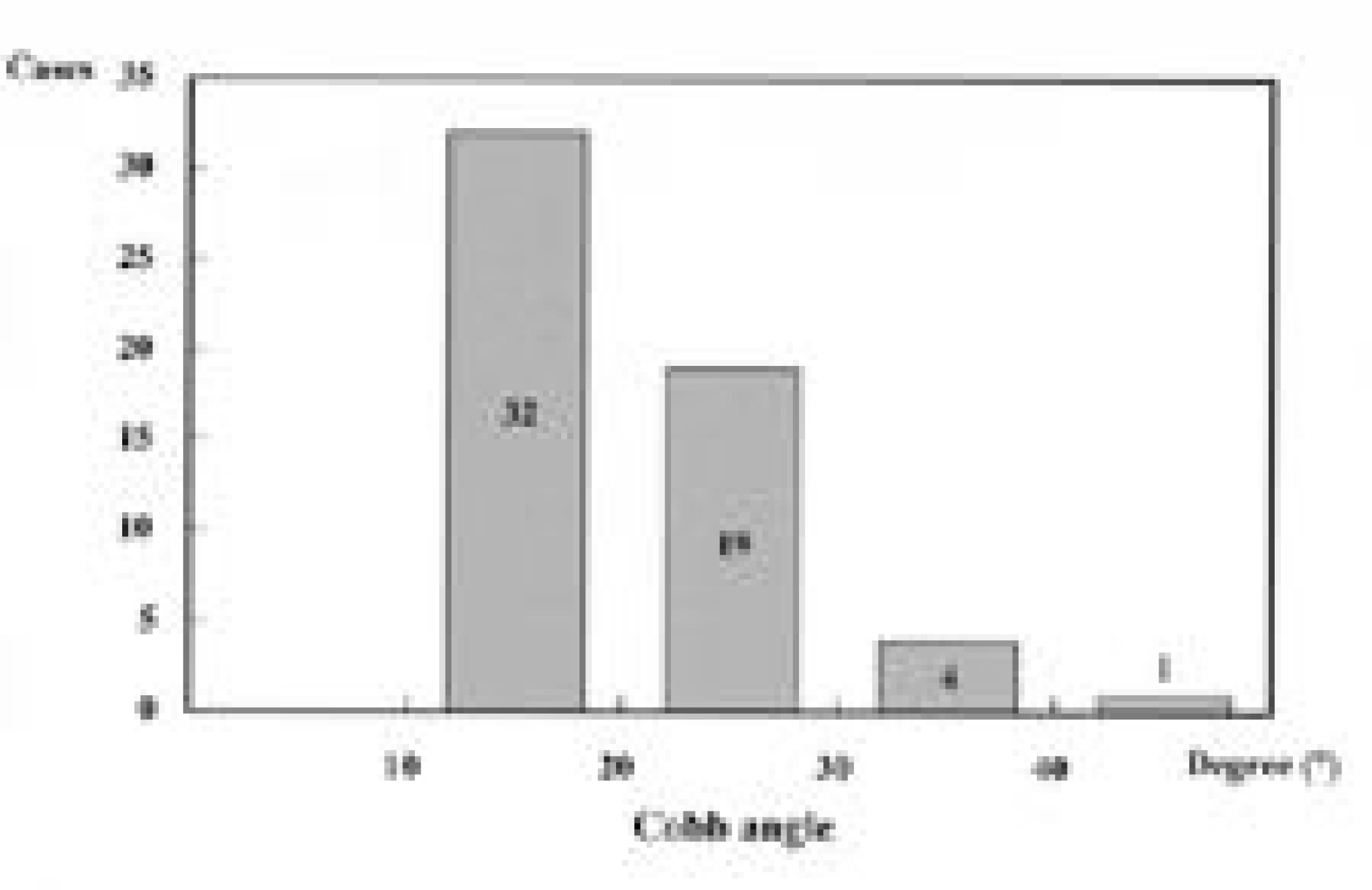
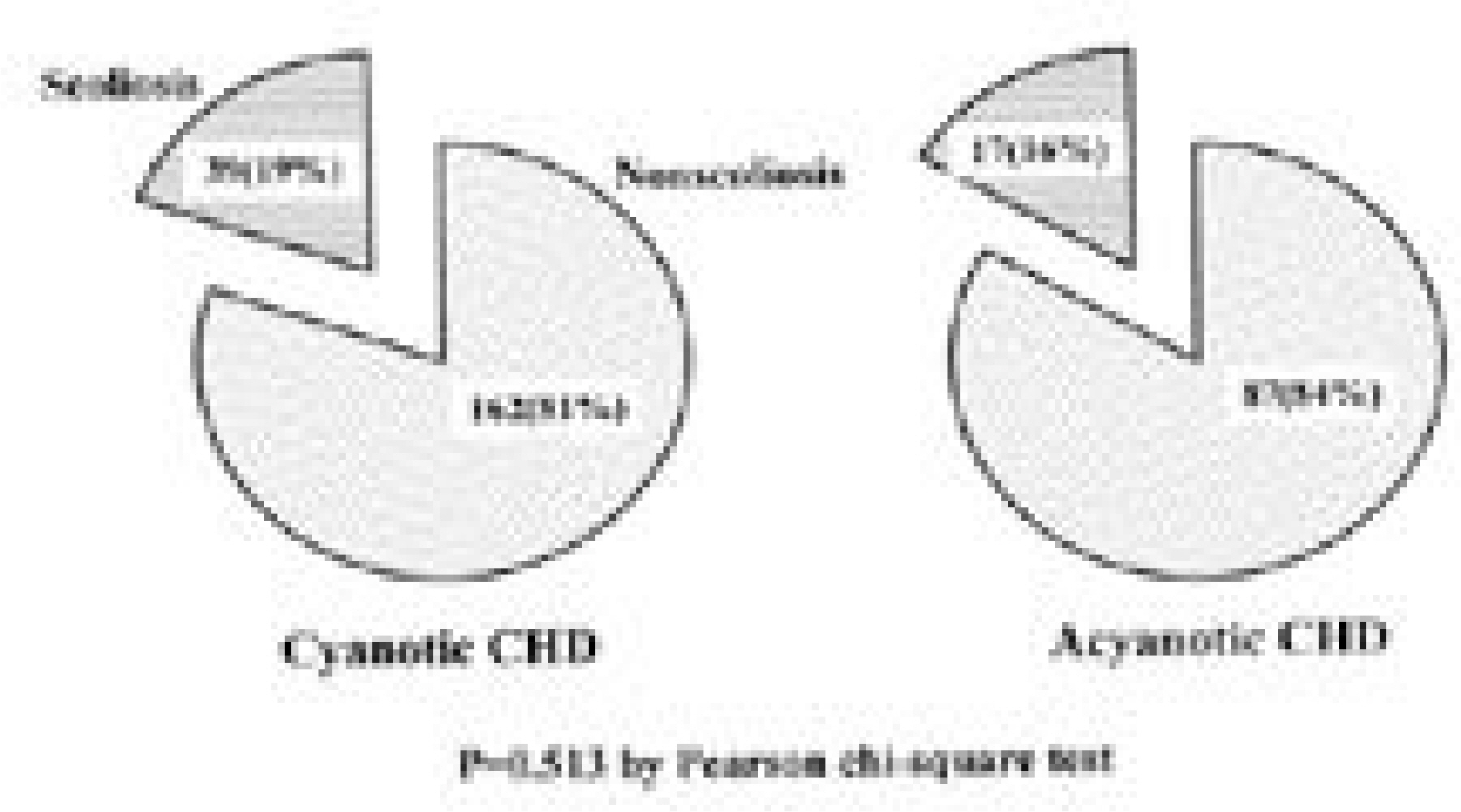
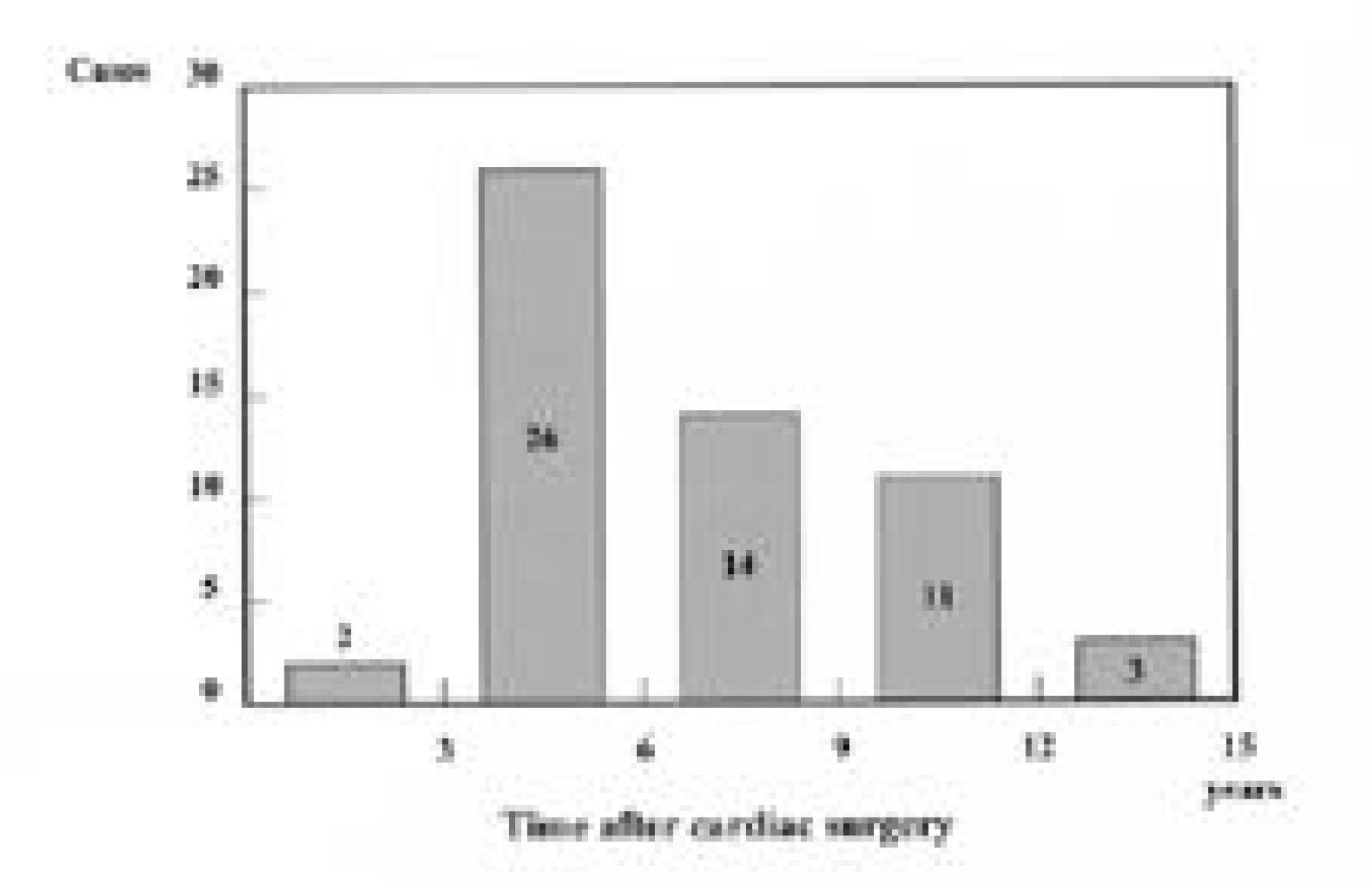
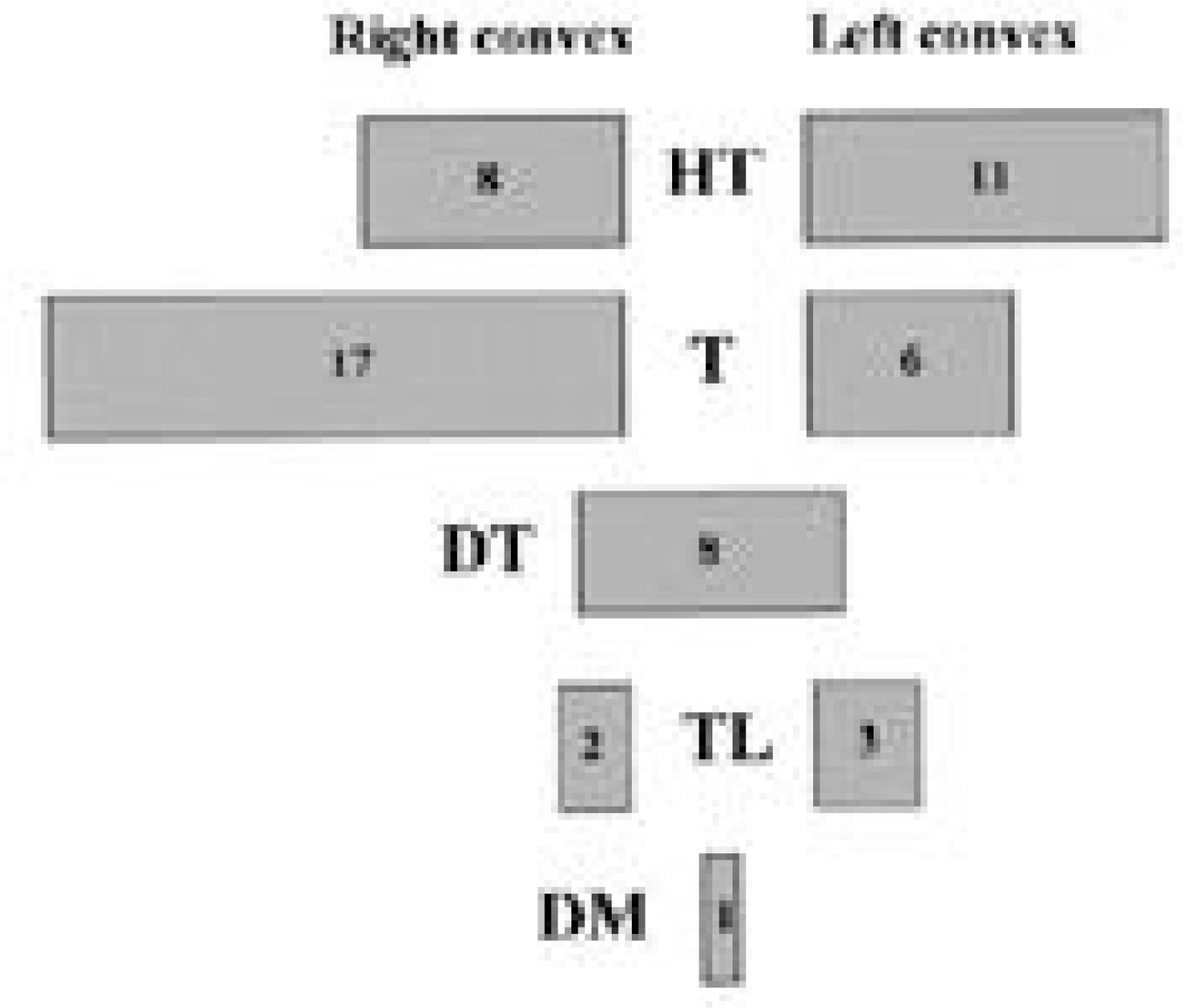
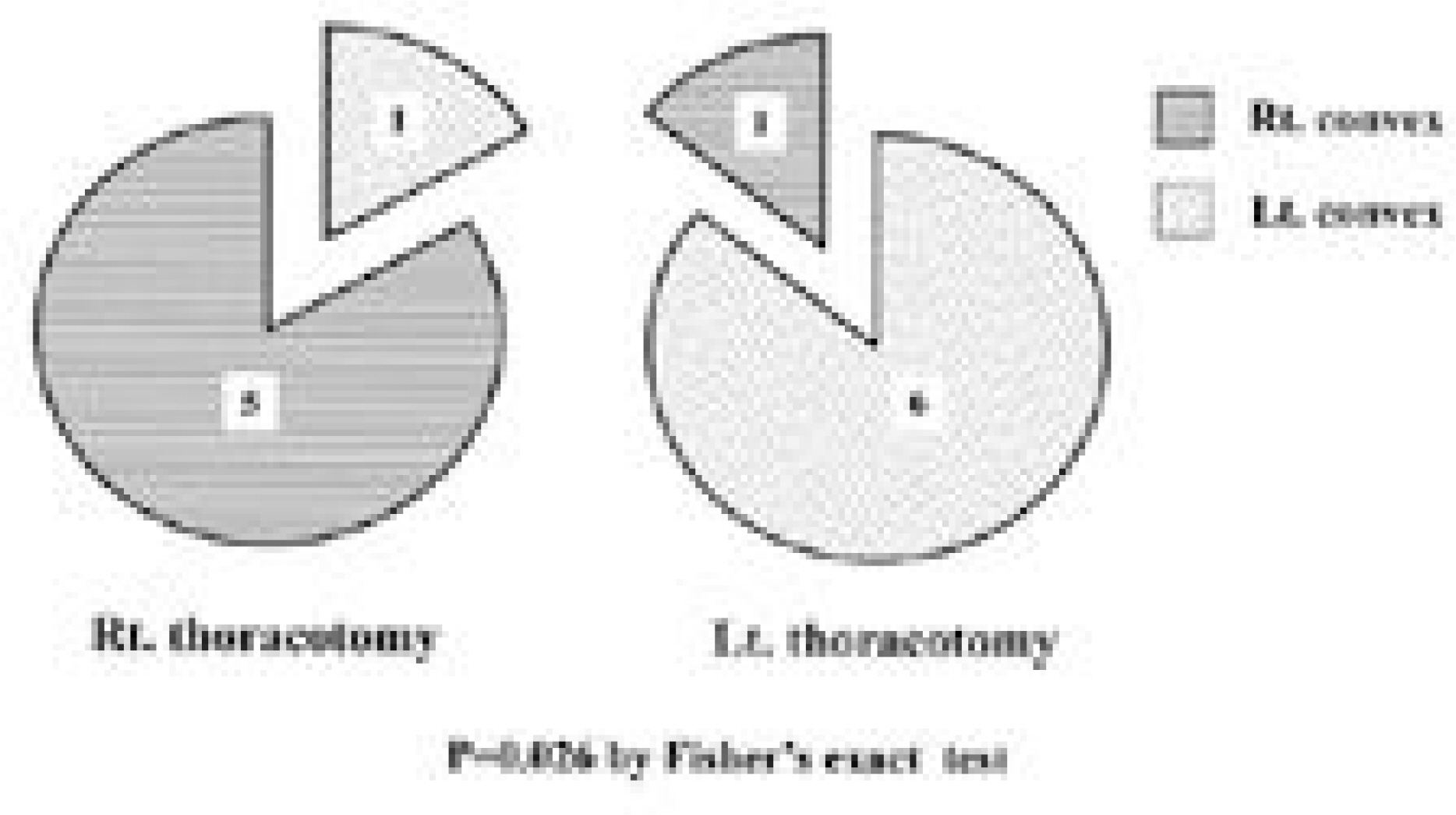
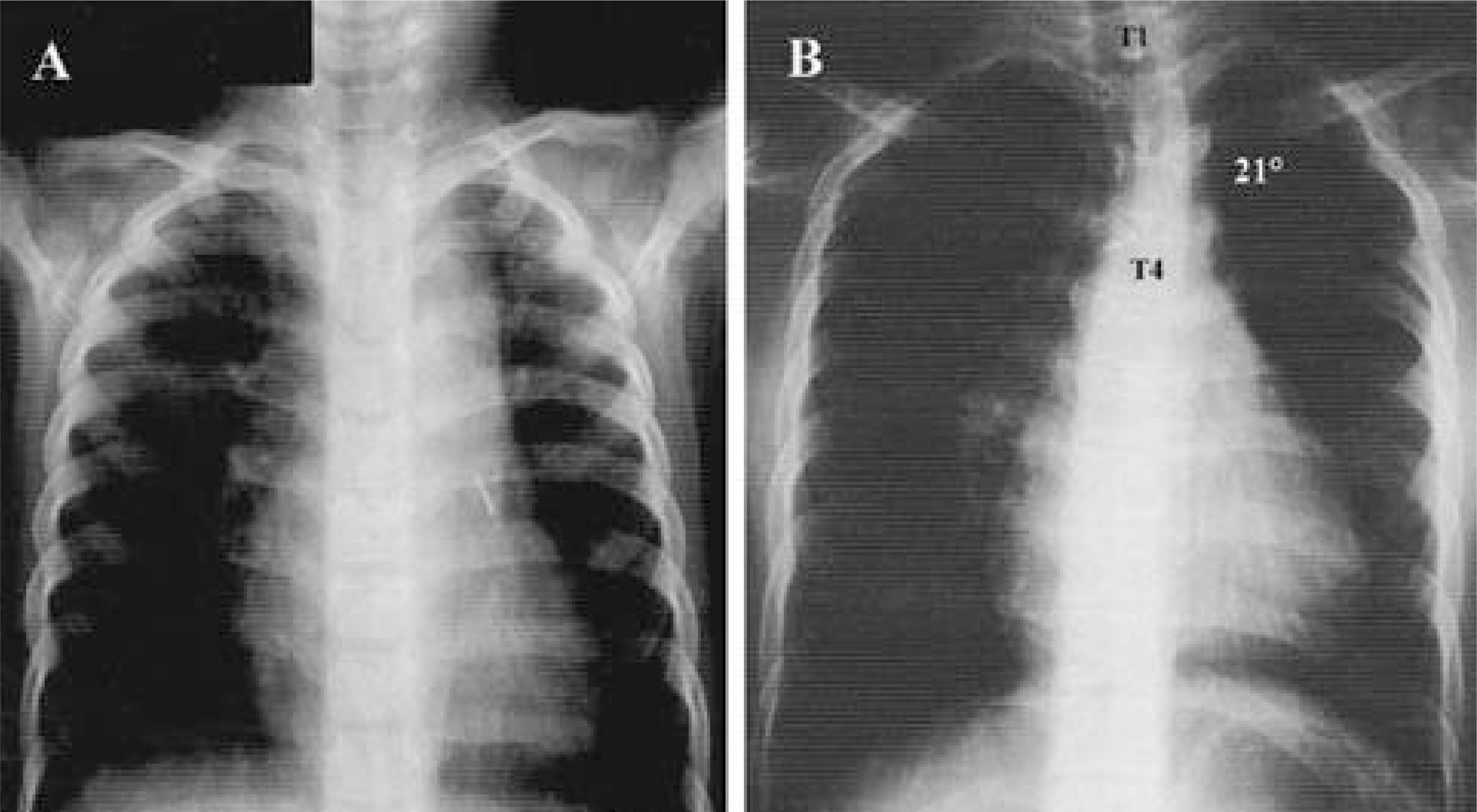
Fifty-six patients(18.4%) had scoliosis of more than 10 degrees. As for location, high thoracic in 19, thoracic in 23, double thoracic in 8, thoraco-lumbar in 5 and double major in 1. Of the 42 patients with thoracic or high thoracic curves, 17(40%) showed convexity to the left. The magnitudes of curves were less than 40 degrees except 1. There was no significant difference in prevalence(p=0.513) and Cobb angle(p=0.634) between cyanosis and acyanosis group. Scoliosis developed between 3 and 6 years after cardiac operation in 26 patients(46%). In high thoracic curve, 6 of 7 patients with left thoracotomy demonstrated convexity to the left and 5 of 6 patients with right thoracotomy demonstrated convexity to the right(p=0.026).
CONCLUSIONS
The prevalence of scoliosis after surgery for congenital heart disease was 18.4%. High thoracic and left thoracic curves were more frequent compared to idiopathic curves and a half of them developed 3 to 6 years after cardiac surgery. Most patients did not have severe curve to need surgical intervention and there was no correlation between severity of scoliosis and age at cardiac operation and cyanosis. In high thoracic curve, the tendency for the curve to be convex to the side of cardiac approach was demonstrated.
Keyword
MeSH Terms
Figure
Reference
-
1). Beals RK, Kenney KH, Lees MH. Congenital heart disease and idiopathic scoliosis. Clin Orthop. 89:112–116. 1972.
Article2). Bisgard JD. Thoracogenic scoliosis: Influence of thoracic disease and thoracic operation on the spine. Arch Surg. 29:417–445. 1934.3). Bisgard JD. Experimental thoracogenic scoliosis. J Tho -rac Surg. 4:435–442. 1935.
Article4). Goldstein LA, Waugh TR. Classification and terminology of scoliosis. Clin Orthop. 93:10–22. 1973.
Article5). Jordan CE, White RI, Fischer KC, Neill C, Dorst JP. The scoliosis of congenital heart disease. Am Heart J. 84:463–469. 1972.
Article6). Kawakami N, Mimatsu K, Deguchi M, Kato F, Maki S. Scoliosis and congenital heart disease. Spine. 20:1252–1256. 1995.
Article7). Luke MJ, McDonnell EJ. Congenital heart disease and scoliosis. J Pediatr. 73:725–733. 1968.
Article8). Reckles LN, Peterson HA, Bianco AJ, Weidman WH. The association of scoliosis and congenital heart disease. J Bone Joint Surg. 75-A:449–455. 1975.9). Roth A, Rosenthal A, Hall JE, Mizel M. Scoliosis and congenital heart disease. Clin Orthop. 93:95–102. 1973.
Article10). Sevastikoglou JA, Aaro S, Lindholm TS, Dahlborn M. experimental scoliosis in growing rabbits by operations on the rib cage. Clin Orthop. 136:282–286. 1978.
Article11). Suk SI, Lee CS, Lee JS, Kim WJ. Scoliosis and congenital heart disease. J Korean Orthop Assoc. 24:221–226. 1989.
Article12). Van Biezen FC, Bakx PAGM, De Villeneuve VH, Hop WC. Scoliosis in children after thoracotomy for aortic coarctation. J Bone Joint Surg. 75-A:514–518. 1993.
Article13). Weinstein SL. The pediatric spine, Principles and prac -tice. New York: Raven Press;1994.14). Wright WD, Niebauer JJ. Congenital heart disease and scoliosis. J Bone Joint Surg. 38-A:1131–1136. 1956.
Article