J Korean Acad Periodontol.
2009 Aug;39(Suppl):287-291.
Clinical presentation of a horse-derived biomaterial and its Biocompatibility: A Clinical Case Report
- Affiliations
-
- 1Department of Periodontology and Dental Research Institute, School of dentistry, Seoul National University, Seoul, Korea. ccpperio@snu.ac.kr
Abstract
-
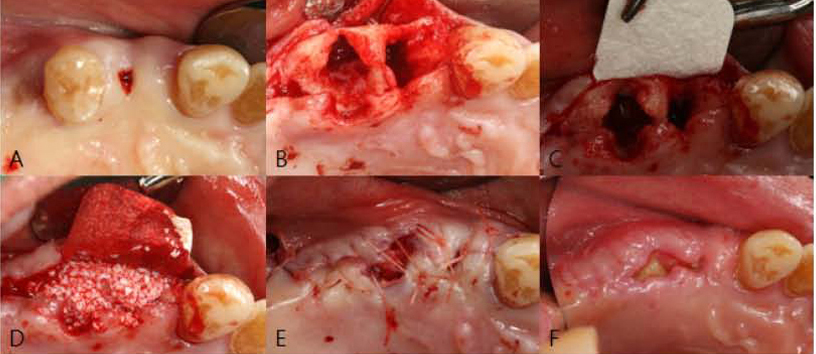
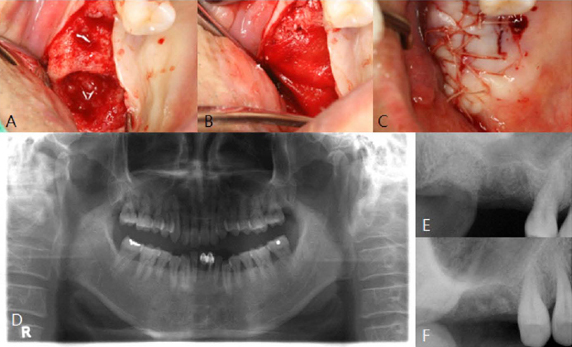
PURPOSE: The objective of this clinical presentation was to present a clinical case series report of socket preservation, sinus augmentation, and bone grafting using a horse-derived biomaterial.
METHODS
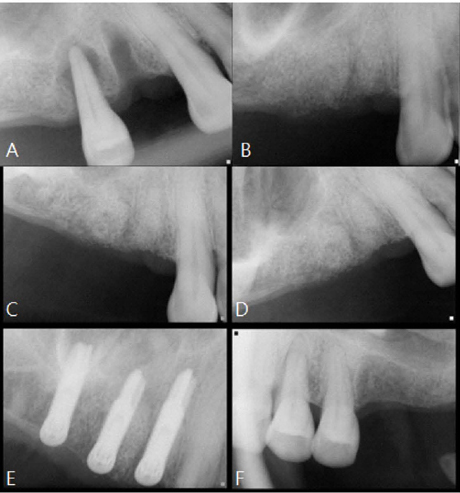
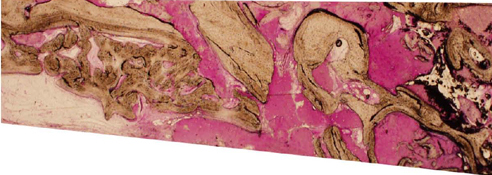
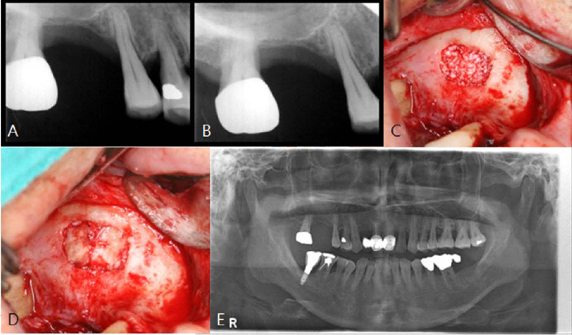
A horse-derived biomaterial was used in 8 patients for different indications including socket preservation following tooth extraction, osseous bone grafting, and sinus augementation procedures. Surgeries were performed by a well trained specialist and clinical radiographs were obtained at designated intervals. Biopsy cores of 2 x 8 mm prior to implant placement was obtained following a healing interval of 4 - 6 months. A clinical and histologic evaluation was performed to evaluate the clinical effectiveness and biocompatibility of the biomaterial.
RESULTS
All surgeries in 8 patients were successful with uneventful healing except for one case with membrane exposure that eventually resulted with a positive outcome. Radiographic display of the healing phase during different intervals showed increased radiopacity of granular nature as the healing time increased. No signs of adverse effect or infection was observed clinically and the tissues surrounding the biomaterial seemed well-tolerated with good intentional healing. The augmented sinuses healed uneventfully suggesting in part, good biocompatibility of the biomaterial. Dental implants placed following socket preservation were inserted with high initial torque suggesting good initial stability and bone quality.
CONCLUSIONS
Our results show that at least on a tentative level, a horse-derived biomaterial may be used clinically in socket preservation, sinus augmentation, bone grafting techniques with good intentional healing and positive results.
MeSH Terms
Figure
Reference
-
1. Jensen SS, Aaboe M, Pinholt EM. Tissue reaction and material characteristics of four bone substitutes. Int J Oral Maxillofac Implants. 1966; 11:55–66.2. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets:Histochemical observations at 9 months. J Periodontol. 2001; 72:152–159.
Article3. Carmagnola D, Adriaens P, Berglundh T. Healing of human extraction sockets filled with Bio-oss. Clin Oral Implants Res. 2003; 14:137–143.4. Wenz B, Oesch B, Horst M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials. 2001; 22:1599–1606.
Article5. Yukna RA, Mayer ET, Brite DV. Longitudinal evaluation of durapatite ceramic as an alloplastic implant in periodontal defects after 3 years. J Periodontol. 1984; 55:633–637.
Article6. Meffert RM, Thomas JR, Hamilton KM, Brownstein CN. Hydroxyapatite as an alloplastic graft in the treatment of human periodontalosseous defects. J Periodontol. 1985; 56:63–73.
Article7. Klinglsberg J. Scleral allograft in the repair of periodontal osseous defects. NY state Dent J. 1972; 38:418–420.8. Radents WH, Collings CK. The implantium of plaster of paris in the alveolar process of the dog. J Periodontol. 1965; 36:357–364.9. Stephan T, Sonis RC, Williams , Marjorie K, Jeffcoat . Healing of spontaneous periodontal defects in dogs treated with xenogenic demineralized bone. J Periodontol. 1979; 50:23–27.10. Pinholt EM, Bang G, Haanaes HR. Alveolar ridge augmentation in rats by Bio-oss. Scand J Dent Res. 1991; 99:154–161.
Article11. Klinge B, Alberius P, Isaksson S, Jonsson AJ. Osseous response to implanted natural bone mineral and synthetic hydroxylapatite ceramic in the repair of experimental skull bone defects. J oral maxillofac Surg. 1992; 50:241–249.
Article12. Young C, Sandstedt P, Skoglund A. A comparative study of anoganic xenogenic bone and autogenous bone implants for bone regeneration in rabbits. Int J oral Maxillofacial Implant. 1999; 14:72–76.13. Skoglund A, Hising P, Young C. A Clinical and histologic examination in humans of the osseous response to implanted natural bone mineral. Int J Oral Maxillofac Implants. 1997; 12:194–199.14. Becker W, Clokie C, Sennerby L, Urist MR, Becker BE. Histologic findings after implantation and evaluation of different grafting materials and titanium micro screws into extraction sockets: case reports. J Periodontol. 1998; 68:414–421.
Article15. Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop Relat Res. 1987; 225:7–15.
Article16. Burchardt H. The biology of bone graft repair. Clin Orthop. 1983; 174:28–32.
Article17. Brown P, Preece MA, Will RG. "Friendly fire" in medicine: hormones, homografts, and Creutzfeldt-Jakob disease in the UK. Lancet. 1992; 340:24–27.
Article18. Yeo Shin-Il, Park Sung-Hwan, Noh Woo-chang, et al. A comparative analysis of basic characteristics of several deproteinized bovine bone substitutes. J Korean Acad Periodontol. 2009; 39:149–156.
Article19. Son Woo-Kyung, Shin Seung-Yun, Yang Seung-Min, Kye Seoung-Beom. Maxillary sinus floor augmentation with anorganic bovine bone: Histologic evaluation in humans. J Korean Acad Periodontol. 2009; 39:95–102.
Article20. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets. Part I: histomorphometric evaluations at 9 months. J Periodontol. 2000; 71:1015–1023.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- From Idea to Product
- An atypical presentation of leiomyosarcoma causing extremity compartment syndrome of the crural region in a Dutch Warmblood mare: a case report
- Socket preservation using deproteinized horse-derived bone mineral
- The Mental Health and Occupational Characteristic of Horse Stable Hand Workers in Korea
- A case of the horse-shoe kidney