World J Mens Health.
2013 Dec;31(3):254-261.
Preliminary Report on the Safety of a New Herbal Formula and Its Effect on Sperm Quality
- Affiliations
-
- 1Catholic Fertility Care Center, Seoul St. Mary's Hospital, The Catholic University of Korea, College of Medicine, Seoul, Korea.
- 2Department of Urology, Seoul St. Mary's Hospital, The Catholic University of Korea, College of Medicine, Seoul, Korea. ksw1227@catholic.ac.kr
- 3Department of Obstetrics and Gynecology, Seoul St. Mary's Hospital, The Catholic University of Korea, College of Medicine, Seoul, Korea.
- 4Korea Bio Medical Science Institute, Seoul, Korea.
- 5Department of Urology, Second Hospital of Lanzhou University, Lanzhou, China.
Abstract
- PURPOSE
Male infertility is a serious problem, and its prevalence has been increasing. Therefore, we investigated the safety of a new herbal formula and its effects on sperm quality.
MATERIALS AND METHODS
An in vitro cytotoxicity test in TM3 Leydig cells was performed to evaluate cell viability after administration of five types of herbs separately and of a new herbal formula containing these five. An in vivo test in male mice was performed to evaluate the influence of the new herbal formula on the reproductive organs and sperm quality. After the 8- and 28-day oral administration of the new herbal formula, the weights of the reproductive organs were measured and the sperm count and motility were evaluated.
RESULTS
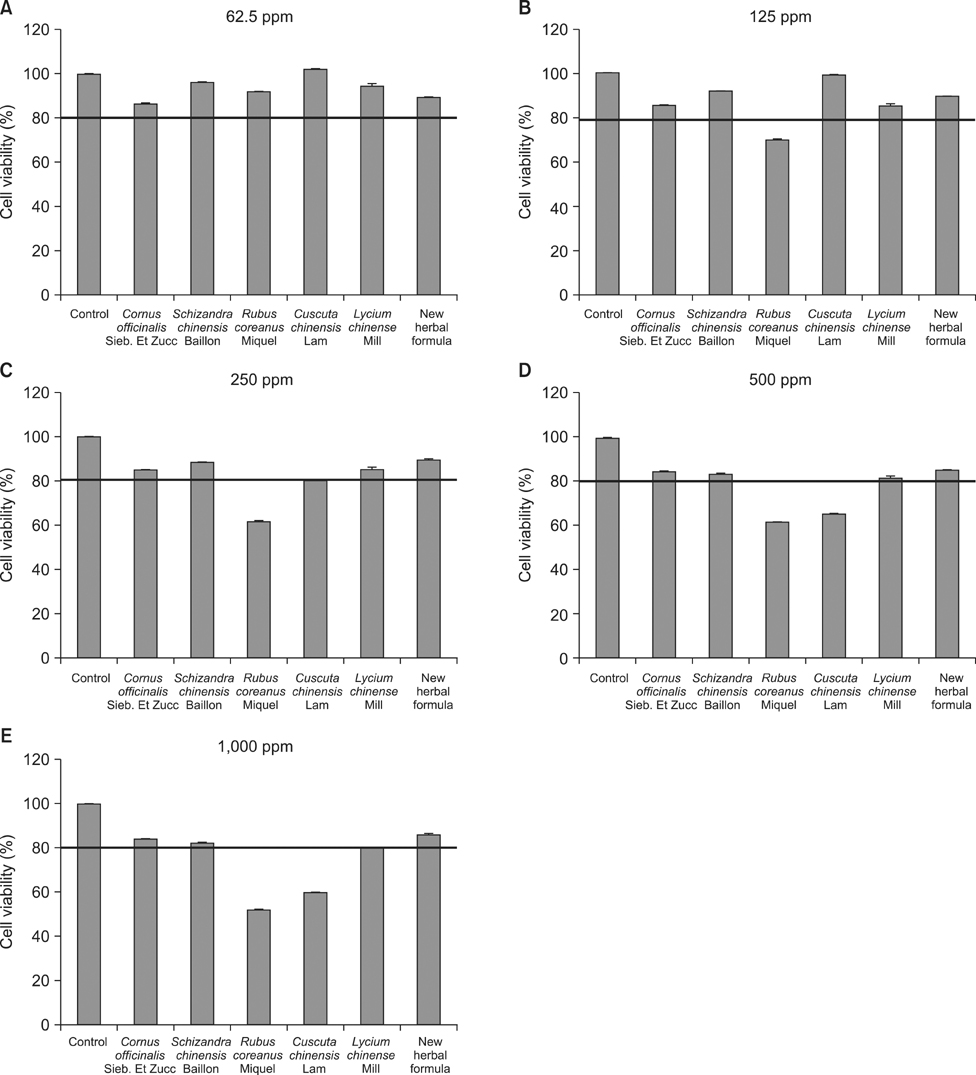
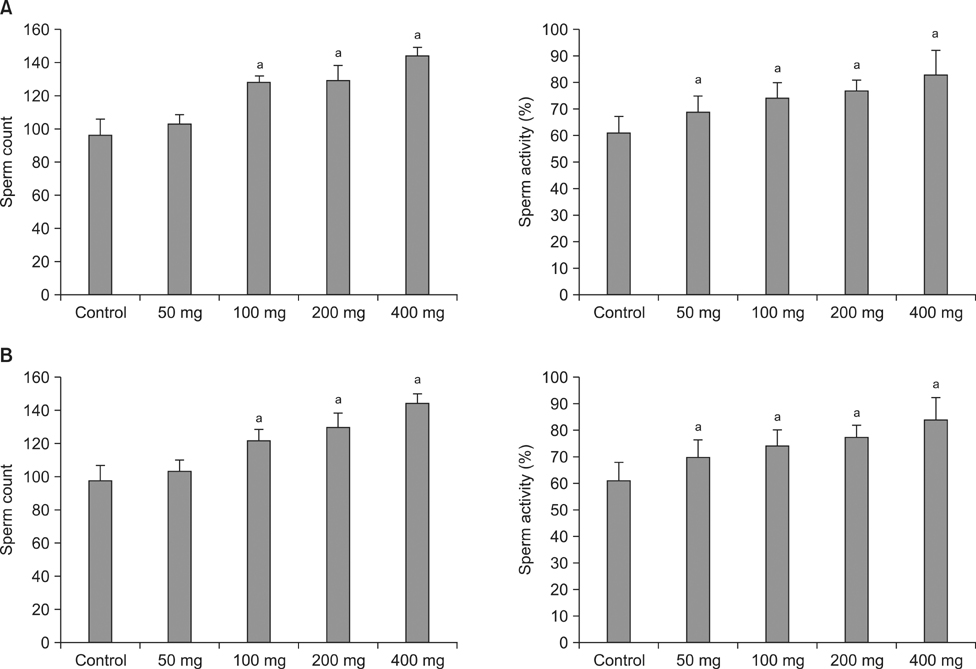
In the in vitro cytotoxicity test, less than 80% cell viability at concentrations of 500 mg/L and 1,000 mg/L of Rubus coreanus Miquel and Cuscuta chinensis Lam was observed. However, more than 80% cell viability was observed at all the tested concentrations of the new herbal formula. After the 8- and 28-day oral administration, there were no considerable changes in body weight. The weights of the testes, epididymis, and seminal vesicles after the 8- and 28-day oral administration were similar to those of the control. The sperm count and activity were significantly improved compared with those of the control group at 8 and 28 days after 100, 200, and 400 mg of oral administration.
CONCLUSIONS
The safety of the new formula and its positive effect on the sperm quality were observed after the oral administration of the formula.
Keyword
MeSH Terms
Figure
Reference
-
1. Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998; 13:Suppl 1. 33–44.
Article2. Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod. 1991; 6:811–816.3. Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertil Steril. 2002; 77:873–882.
Article4. Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, et al. European Association of Urology Working Group on Male Infertility. European Association of Urology guidelines on male infertility: the 2012 update. Eur Urol. 2012; 62:324–332.
Article5. Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008; 14:243–258.
Article6. Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000; 21:33–44.7. Comhaire FH, El Garem Y, Mahmoud A, Eertmans F, Schoonjans F. Combined conventional/antioxidant "Astaxanthin" treatment for male infertility: a double blind, randomized trial. Asian J Androl. 2005; 7:257–262.
Article8. Scott R, MacPherson A, Yates RW, Hussain B, Dixon J. The effect of oral selenium supplementation on human sperm motility. Br J Urol. 1998; 82:76–80.
Article9. Vicari E, Calogero AE. Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Hum Reprod. 2001; 16:2338–2342.
Article10. Liu F, Ng TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000; 66:725–735.
Article11. Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 2000; 66:709–723.
Article12. Lee NH, Seo CS, Lee HY, Jung DY, Lee JK, Lee JA, et al. Hepatoprotective and Antioxidative Activities of Cornus officinalis against Acetaminophen-Induced Hepatotoxicity in Mice. Evid Based Complement Alternat Med. 2012; 2012:804924.13. Cheng N, Ren N, Gao H, Lei X, Zheng J, Cao W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem Toxicol. 2013; 55:234–240.
Article14. Bhandary B, Lee HY, Back HI, Park SH, Kim MG, Kwon JW, et al. Immature rubus coreanus shows a free radical-scavenging effect and inhibits cholesterol synthesis and secretion in liver cells. Indian J Pharm Sci. 2012; 74:211–216.
Article15. Yen FL, Wu TH, Lin LT, Lin CC. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J Ethnopharmacol. 2007; 111:123–128.
Article16. Yin JY, Nie SP, Zhou C, Wan Y, Xie MY. Chemical characteristics and antioxidant activities of polysaccharide purified from the seeds of Plantago asiatica L. J Sci Food Agric. 2010; 90:210–217.17. Lin CC, Chuang SC, Lin JM, Yang JJ. Evaluation of the anti-inflammatory hepatoprotective and antioxidant activities of Lycium chinense from Taiwan. Phytomedicine. 1997; 4:213–220.
Article18. Ayaki M, Iwasawa A, Niwano Y. In vitro assessment of the cytotoxicity of six topical antibiotics to four cultured ocular surface cell lines. Biocontrol Sci. 2012; 17:93–99.
Article19. Bae SH, Che JH, Seo JM, Jeong J, Kim ET, Lee SW, et al. In vitro biocompatibility of various polymer-based microelectrode arrays for retinal prosthesis. Invest Ophthalmol Vis Sci. 2012; 53:2653–2657.
Article20. Yang J, Liu Y, Wang H, Liu L, Wang W, Wang C, et al. The biocompatibility of fatty acid modified dextran-agmatine bioconjugate gene delivery vector. Biomaterials. 2012; 33:604–613.
Article21. Dreikorn K. Complementary and alternative medicine in urology. BJU Int. 2005; 96:1177–1184.
Article22. Cheong Y, Nardo LG, Rutherford T, Ledger W. Acupuncture and herbal medicine in in vitro fertilisation: a review of the evidence for clinical practice. Hum Fertil (Camb). 2010; 13:3–12.23. Jellin JM. Natural medicines comprehensive database/compiled by the editors of prescriber's letter, pharmacist's letter. 4th ed. Stockton, CA: Therapeutic Research Faculty;2002.24. Gurley BJ. Clinical pharmacology and dietary supplements: an evolving relationship. Clin Pharmacol Ther. 2010; 87:235–238.
Article25. de Lamirande E, Gagnon C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic Biol Med. 1993; 14:157–166.26. Zorn B, Vidmar G, Meden-Vrtovec H. Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl. 2003; 26:279–285.
Article27. Zalata AA, Ahmed AH, Allamaneni SS, Comhaire FH, Agarwal A. Relationship between acrosin activity of human spermatozoa and oxidative stress. Asian J Androl. 2004; 6:313–318.28. Jedrzejczak P, Fraczek M, Szumała-Kakol A, Taszarek-Hauke G, Pawelczyk L, Kurpisz M. Consequences of semen inflammation and lipid peroxidation on fertilization capacity of spermatozoa in in vitro conditions. Int J Androl. 2005; 28:275–283.
Article29. Villegas J, Schulz M, Soto L, Iglesias T, Miska W, Sánchez R. Influence of reactive oxygen species produced by activated leukocytes at the level of apoptosis in mature human spermatozoa. Fertil Steril. 2005; 83:808–810.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of a Herbal Formula that Mainly Consists of Cornus Officinalis for Erectile Dysfunction: A Double-blind, Placebo-controlled Study
- Efficacy and safety of an herbal formula (KBMSI-2) in the treatment of erectile dysfunction: A preliminary clinical study
- Comparison of measurement of human sperm motility by sperm quality analyzer and makler counting chamber
- Supplementation of cryoprotective extender with resveratrol decreases apoptosis index and reactive oxygen species levels in post-thaw dog sperm
- Ginseng for Improving Semen Quality Parameters: A Systematic Review