Tuberc Respir Dis.
2014 Aug;77(2):73-80. 10.4046/trd.2014.77.2.73.
Vitamin D Inhibits Expression and Activity of Matrix Metalloproteinase in Human Lung Fibroblasts (HFL-1) Cells
- Affiliations
-
- 1Department of Internal Medicine, Wonkwang University Sanbon Hospital, Wonkwang University College of Medicine, Gunpo, Korea. hikim7337@gmail.com
- KMID: 2320542
- DOI: http://doi.org/10.4046/trd.2014.77.2.73
Abstract
- BACKGROUND
Low levels of serum vitamin D is associated with several lung diseases. The production and activation of matrix metalloproteinases (MMPs) may play an important role in the pathogenesis of emphysema. The aim of the current study therefore is to investigate if vitamin D modulates the expression and activation of MMP-2 and MMP-9 in human lung fibroblasts (HFL-1) cells.
METHODS
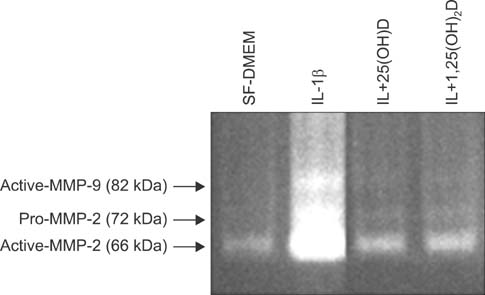
HFL-1 cells were cast into three-dimensional collagen gels and stimulated with or without interleukin-1beta (IL-1beta) in the presence or absence of 100 nM 25-hydroxyvitamin D (25(OH)D) or 1,25-dihydroxyvitamin D (1,25(OH)2D) for 48 hours. Trypsin was then added into the culture medium in order to activate MMPs. To investigate the activity of MMP-2 and MMP-9, gelatin zymography was performed. The expression of the tissue inhibitor of metalloproteinase (TIMP-1, TIMP-2) was measured by enzyme-linked immunosorbent assay. Expression of MMP-9 mRNA and TIMP-1, TIMP-2 mRNA was quantified by real time reverse transcription polymerase chain reaction.
RESULTS
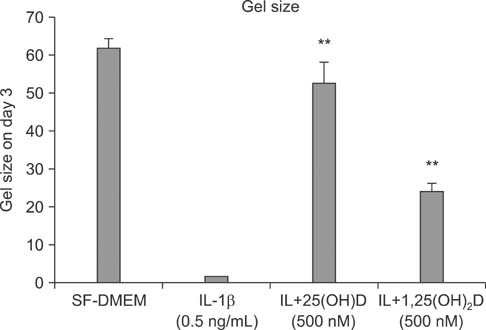
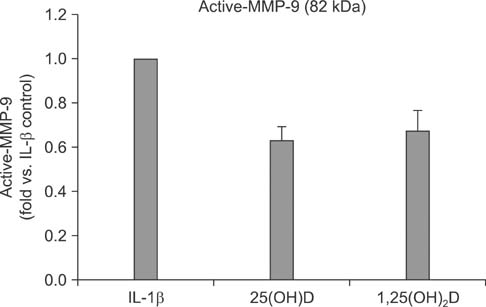
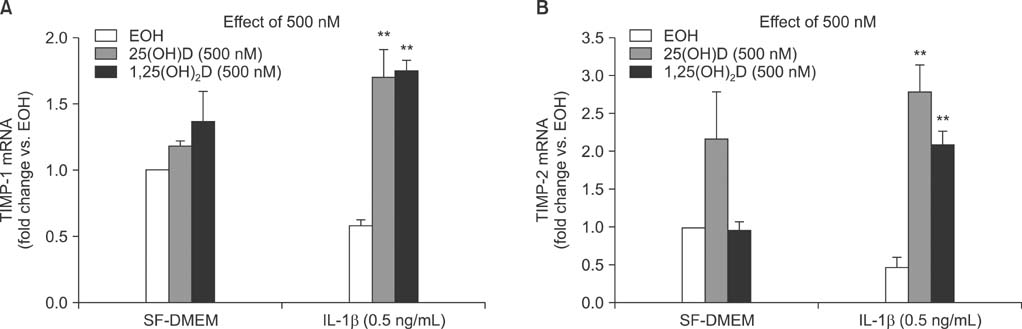
IL-1beta significantly stimulated MMP-9 production and mRNA expression. Trypsin converted latent MMP-2 and MMP-9 into their active forms of MMP-2 (66 kDa) and MMP-9 (82 kDa) within 24 hours. This conversion was significantly inhibited by 25(OH)D (100 nM) and 1,25(OH)2D (100 nM). The expression of MMP-9 mRNA was also significantly inhibited by 25(OH)D and 1,25(OH)2D.
CONCLUSION
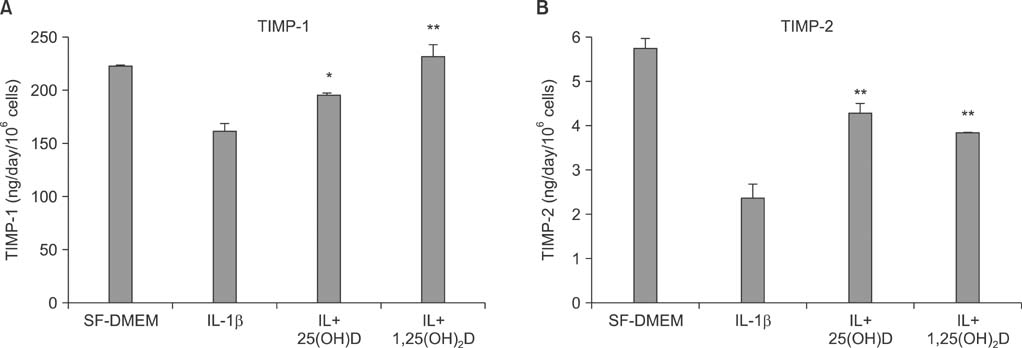
Vitamin D, 25(OH)D, and 1,25(OH)2D play a role in regulating human lung fibroblast functions in wound repair and tissue remodeling through not only inhibiting IL-1beta stimulated MMP-9 production and conversion to its active form but also inhibiting IL-1beta inhibition on TIMP-1 and TIMP-2 production.
Keyword
MeSH Terms
-
Collagen
Emphysema
Enzyme-Linked Immunosorbent Assay
Fibroblasts*
Gelatin
Gels
Humans
Interleukin-1beta
Lung Diseases
Lung*
Matrix Metalloproteinase 9
Matrix Metalloproteinases
Polymerase Chain Reaction
Reverse Transcription
RNA, Messenger
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Trypsin
Vitamin D*
Wounds and Injuries
Collagen
Gelatin
Gels
Interleukin-1beta
Matrix Metalloproteinase 9
Matrix Metalloproteinases
RNA, Messenger
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Trypsin
Vitamin D
Figure
Reference
-
1. Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983; 221:1181–1183.2. Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1 alpha,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: effects on immunoglobulin production. J Immunol. 1986; 136:2734–2740.3. Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007; 120:1031–1035.4. Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010; 181:699–704.5. Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005; 128:3792–3798.6. Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972; 54:626–637.7. Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three-dimensional collagen gel matrix. In Vitro Cell Dev Biol Anim. 1996; 32:427–433.8. Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem. 1963; 35:1961–1965.9. Edwards CA, O'Brien WD Jr. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980; 104:161–167.10. Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem. 1994; 218:325–329.11. Zhang Y, McCluskey K, Fujii K, Wahl LM. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J Immunol. 1998; 161:3071–3076.12. Snider GL, Failing LJ, Rennard SI. Chronic bronchitis and emphysema. In : Murray JF, Nadel JA, editors. Textbook of respiratory medicine. 2nd ed. Philadelphia: W.B. Saunders;1994. p. 1331–1397.13. Niewoehner DE. Anatomic and pathophysiological correlations in COPD. In : Baum GL, Crapo JD, Celli BR, Karlinsky JB, editors. Textbook of pulmonary diseases. Philadelphia: Lippincott-Raven;1998. p. 823–842.14. Murphy G, Reynolds JJ, Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-O-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985; 260:3079–3083.15. Eberhardt W, Beeg T, Beck KF, Walpen S, Gauer S, Bohles H, et al. Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int. 2000; 57:59–69.16. Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991; 5:2145–2154.17. Dinarello CA. The biology of interleukin-1. Chem Immunol. 1992; 51:1–32.18. Togo S, Holz O, Liu X, Sugiura H, Kamio K, Wang X, et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am J Respir Crit Care Med. 2008; 178:248–260.19. Rennard SI, Wachenfeldt K. Rationale and emerging approaches for targeting lung repair and regeneration in the treatment of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2011; 8:368–375.20. Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979; 76:1274–1278.21. Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994; 124:401–404.22. Dans MJ, Isseroff R. Inhibition of collagen lattice contraction by pentoxifylline and interferon-alpha, -beta, and -gamma. J Invest Dermatol. 1994; 102:118–121.23. Zhang HY, Gharaee-Kermani M, Phan SH. Regulation of lung fibroblast alpha-smooth muscle actin expression, contractile phenotype, and apoptosis by IL-1beta. J Immunol. 1997; 158:1392–1399.24. Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010; 65:215–220.25. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357:266–281.26. Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009; 179:630–636.27. Gilbert CR, Arum SM, Smith CM. Vitamin D deficiency and chronic lung disease. Can Respir J. 2009; 16:75–80.28. Tsiligianni IG, van der Molen T. A systematic review of the role of vitamin insufficiencies and supplementation in COPD. Respir Res. 2010; 11:171.29. Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000; 223:230–233.30. Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010; 21:375–384.31. Sundar IK, Hwang JW, Wu S, Sun J, Rahman I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem Biophys Res Commun. 2011; 406:127–133.32. Bahar-Shany K, Ravid A, Koren R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J Cell Physiol. 2010; 222:729–737.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Vitamin C, Silicon and Iron on Collagen Synthesis and Break-Down Enzyme Expression in the Human Dermal Fibroblast Cell (HS27)
- Effect of Curcumin on Cancer Invasion and Matrix Metalloproteinase-9 Activity in MDA-MB-231 Human Breast Cancer Cell
- EGCC inhibits tumor growth by inbibiting Matrix Metalloproteinase-9 induction in UM-SCC-1 cells
- Effects of Hepatocyte Growth Factor on Collagen Synthesis and Matrix Metalloproteinase Production in Keloids
- Expression of mRNA for matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival and periodontal ligament fibroblasts treated with lipopolysaccharide from Prevotella intermedia