Tuberc Respir Dis.
2012 Feb;72(2):132-139.
Overexpression of Periostin Protein in Non-Small Cell Lung Carcinoma is Not Related with Clinical Prognostic Significance
- Affiliations
-
- 1Department of Pathology, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea. cnlee@pusan.ac.kr
- 2Department of Physiology, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
- 3Department of Internal Medicine, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea. leemk@pusan.ac.kr
- 4Department of Thoracic Surgery, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
- 5Medical Research Institute, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
Abstract
- BACKGROUND
Periostin is preferentially expressed in periosteum, indicating a potential role in bone formation. Recently, there have been emerging controversies about its role in invasion and metastasis of human malignancies. We attempted to determine the clinicopathological significance of periostin expression in non-small cell lung carcinoma (NSCLC).
METHODS
Immunohistochemical staining of periostin protein from 91 cases of NSCLCs was performed using tissue microarray blocks. The results were correlated with clinicopathological parameters.
RESULTS
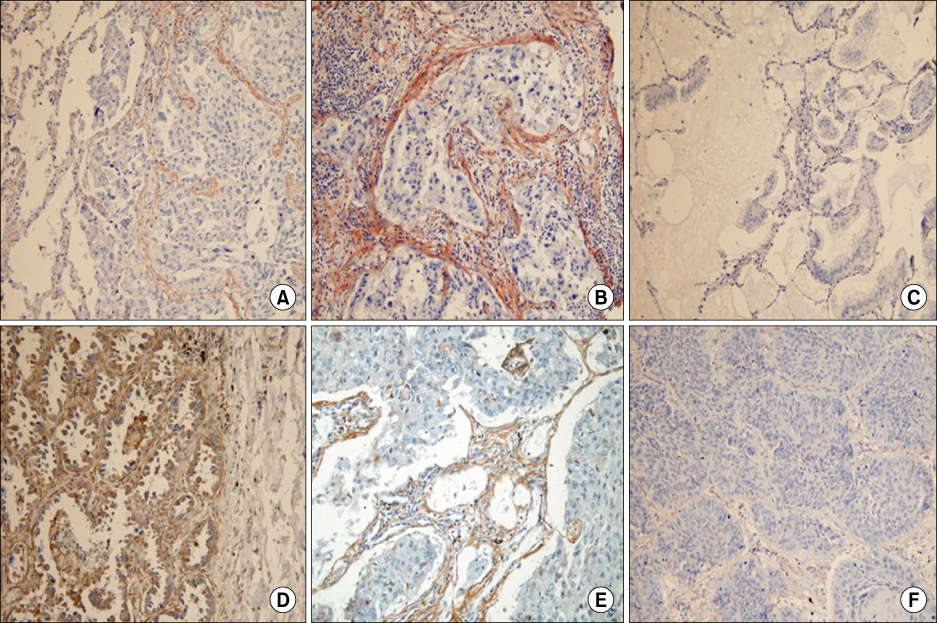
Positive reaction to periostin was predominantly noted in the tumor stroma. The strongest reaction presented as a band-like pattern just around the tumor nests. Non-neoplastic lung tissue and most in-situ carcinomas did not show a positive reaction in their stroma. With respect to tumor differentiation, moderate to poor differentiated tumors (47/77) revealed even higher periostin expression than the well-differentiated ones (4/14) (p=0.024). High periostin expression was positively correlated with E-cadherin and p53 expression, but was not related with patient age, sex, tumor type, PCNA index, b-catenin, cyclin D1, pTNM-T, pTNM-N, stage, and patient survival (p>0.05).
CONCLUSION
These results suggest that periostin might play a role during the biological progression of NSCLC, but may not be related to the clinical prognostic parameters.
MeSH Terms
Figure
Reference
-
1. Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999. 14:1239–1249.2. Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007. 13:962–969.3. Lie-Venema H, Eralp I, Markwald RR, van den Akker NM, Wijffels MC, Kolditz DP, et al. Periostin expression by epicardium-derived cells is involved in the development of the atrioventricular valves and fibrous heart skeleton. Differentiation. 2008. 76:809–819.4. Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR. Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci. 2008. 1123:30–40.5. Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007. 101:695–711.6. Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006. 118:98–104.7. Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004. 5:329–339.8. Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, et al. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer. 2006. 95:1396–1403.9. Kudo Y, Siriwardena BS, Hatano H, Ogawa I, Takata T. Periostin: novel diagnostic and therapeutic target for cancer. Histol Histopathol. 2007. 22:1167–1174.10. Puglisi F, Puppin C, Pegolo E, Andreetta C, Pascoletti G, D'Aurizio F, et al. Expression of periostin in human breast cancer. J Clin Pathol. 2008. 61:494–498.11. Kudo Y, Ogawa I, Kitajima S, Kitagawa M, Kawai H, Gaffney PM, et al. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 2006. 66:6928–6935.12. Kim CJ, Yoshioka N, Tambe Y, Kushima R, Okada Y, Inoue H. Periostin is down-regulated in high grade human bladder cancers and suppresses in vitro cell invasiveness and in vivo metastasis of cancer cells. Int J Cancer. 2005. 117:51–58.13. Sasaki H, Lo KM, Chen LB, Auclair D, Nakashima Y, Moriyama S, et al. Expression of Periostin, homologous with an insect cell adhesion molecule, as a prognostic marker in non-small cell lung cancers. Jpn J Cancer Res. 2001. 92:869–873.14. Sobin LH, Wittekind C. International Union against Cancer. TNM Classification of malignant tumours. 1997. 5th ed. New York: Wiley-Liss.15. Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E, Sobin LH. Histological typing of lung and pleural tumours. International histological classification of tumours. 1999. 3rd ed. Berlin: Springer-Verlag.16. Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008. 18:27–34.17. Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001. 1:46–54.18. Radisky D, Hagios C, Bissell MJ. Tumors are unique organs defined by abnormal signaling and context. Semin Cancer Biol. 2001. 11:87–95.19. López-Maury L, Marguerat S, Bähler J. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat Rev Genet. 2008. 9:583–593.20. Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993. 294:271–278.21. Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, et al. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007. 26:2082–2094.22. Sasaki H, Dai M, Auclair D, Fukai I, Kiriyama M, Yamakawa Y, et al. Serum level of the periostin, a homologue of an insect cell adhesion molecule, as a prognostic marker in nonsmall cell lung carcinomas. Cancer. 2001. 92:843–848.23. Ouyang G, Liu M, Ruan K, Song G, Mao Y, Bao S. Upregulated expression of periostin by hypoxia in non-small-cell lung cancer cells promotes cell survival via the Akt/PKB pathway. Cancer Lett. 2009. 281:213–219.24. Hong L, Sun H, Lv X, Yang D, Zhang J, Shi Y. Expression of periostin in the serum of NSCLC and its function on proliferation and migration of human lung adenocarcinoma cell line (A549) in vitro. Mol Biol Rep. 2010. 37:2285–2293.25. Soltermann A, Tischler V, Arbogast S, Braun J, Probst-Hensch N, Weder W, et al. Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res. 2008. 14:7430–7437.26. Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, et al. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004. 24:3992–4003.27. Yan W, Shao R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation. J Biol Chem. 2006. 281:19700–19708.28. Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3' kinase/AKT pathways. Oncogene. 2005. 24:7443–7454.29. Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol. 2007. 18:1605–1619.30. Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991. 251:1451–1455.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Expression and Prognostic Value of VEGF, HIF-1alpha, EGFR in Non-Small Cell Lung Cancer
- The Combination of Periostin Overexpression and Microvascular Invasion Is Related to a Poor Prognosis for Hepatocellular Carcinoma
- Prognostic significance of p53 protein expression of primary non-small cell lung cancer
- A Study of the Prognostic Factors in Resected Stage I Non-Small Cell Lung Cancer
- Expression of Survivin in Non-Small Cell Lung Carcinoma: Relationship to Tumor Biology and Prognosis in Surgically Treated Patients