Tuberc Respir Dis.
2011 Sep;71(3):202-209.
CT Radiologic Findings in Patients with Tuberculous Destroyed Lung and Correlation with Lung Function
- Affiliations
-
- 1Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea. jcy2475@dsmc.or.kr
- 2Department of Radiology, Keimyung University School of Medicine, Daegu, Korea.
Abstract
- BACKGROUND
A tuberculous destroyed lung is sequelae of pulmonary tuberculosis and causes various respiratory symptoms and pulmonary dysfunction. The patients with a tuberculous destroyed lung account for a significant portion of those with chronic lung disease in Korea. However, few reports can be found in the literature. We investigated the computed tomography (CT) findings in a tuberculous destroyed lung and the correlation with lung function.
METHODS
A retrospective analysis was carried out for 44 patients who were diagnosed with a tuberculous destroyed lung at the Keimyung University Dongsan Hospital between January 2004 and December 2009.
RESULTS
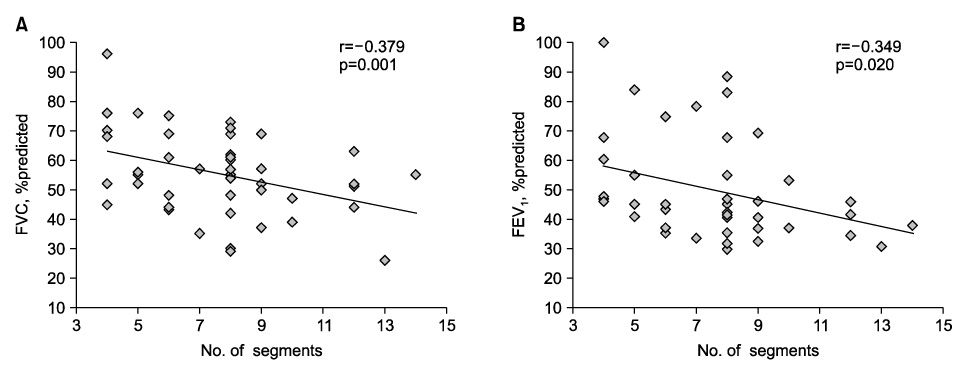
A chest CT scan showed various thoracic sequelae of tuberculosis. In lung parenchymal lesions, there were cicatrization atelectasis in 37 cases (84.1%) and emphysema in 13 cases. Bronchiectasis (n=39, 88.6%) was most commonly found in airway lesions. The mean number of destroyed bronchopulmonary segments was 7.7 (range, 4~14). The most common injured segment was the apicoposterior segment of the left upper lobe (n=36, 81.8%). In the pulmonary function test, obstructive ventilatory defects were observed in 31 cases (70.5%), followed by a mixed (n=7) and restrictive ventilatory defect (n=5). The number of destroyed bronchopulmonary segments showed a significant negative correlation with forced vital capacity (FVC), % predicted (r=-0.379, p=0.001) and forced expiratory volume in one second (FEV1), % predicted (r=-0.349, p=0.020). After adjustment for age and smoking status (pack-years), the number of destroyed segments also showed a significant negative correlation with FVC, % predicted (B=-0.070, p=0.014) and FEV1, % predicted (B=-0.050, p=0.022).
CONCLUSION
Tuberculous destroyed lungs commonly showed obstructive ventilatory defects, possibly due to bronchiectasis and emphysema. There was negative correlation between the extent of destruction and lung function.
Keyword
MeSH Terms
Figure
Reference
-
1. Tuberculosis (TB): global tuberculosis control 2010. World Health Organization (WHO). c2011. cited 2011 Sep 16. Geneva, Switzerland: WHO;Available from: http://www.who.int/tb/publications/global_report/2010/en.2. Bobrowitz ID, Rodescu D, Marcus H, Abeles H. The destroyed tuberculous lung. Scand J Respir Dis. 1974. 55:82–88.3. Lee JH, Chang JH. Lung function in patients with chronic airflow obstruction due to tuberculous destroyed lung. Respir Med. 2003. 97:1237–1242.4. Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med. 1989. 83:195–198.5. Snider GL, Doctor L, Demas TA, Shaw AR. Obstructive airway disease in patients with treated pulmonary tuberculosis. Am Rev Respir Dis. 1971. 103:625–640.6. Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000. 55:32–38.7. Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, et al. Pulmonary impairment after tuberculosis. Chest. 2007. 131:1817–1824.8. Plit ML, Anderson R, Van Rensburg CE, Page-Shipp L, Blott JA, Fresen JL, et al. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J. 1998. 12:351–356.9. Lam KB, Jiang CQ, Jordan RE, Miller MR, Zhang WS, Cheng KK, et al. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest. 2010. 137:593–600.10. Lee SW, Kim YS, Kim DS, Oh YM, Lee SD. The risk of obstructive lung disease by previous pulmonary tuberculosis in a country with intermediate burden of tuberculosis. J Korean Med Sci. 2011. 26:268–273.11. Lee BH, Kim YS, Lee KD, Lee JH, Kim SH. Health-related quality of life measurement with St. George's respiratory questionnaire in post-tuberculous destroyed lung. Tuberc Respir Dis. 2008. 65:183–190.12. Ryu YJ, Lee JH, Chun EM, Chang JH, Shim SS. Clinical outcomes and prognostic factors in patients with tuberculous destroyed lung. Int J Tuberc Lung Dis. 2011. 15:246–250.13. Kim HY, Song KS, Goo JM, Lee JS, Lee KS, Lim TH. Thoracic sequelae and complications of tuberculosis. Radiographics. 2001. 21:839–858.14. American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991. 144:1202–1218.15. The global initiative for chronic obstructive lung disease (GOLD). GOLD [Homepage]. c2010-2011. cited 2011 Sep 17. GOLD;Available from: http://www.goldcopd.org.16. Long R, Maycher B, Dhar A, Manfreda J, Hershfield E, Anthonisen N. Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function. Chest. 1998. 113:933–943.17. Im JG, Itoh H, Lee KS, Han MC. CT-pathology correlation of pulmonary tuberculosis. Crit Rev Diagn Imaging. 1995. 36:227–285.18. Ashour M, Pandya L, Mezraqji A, Qutashat W, Desouki M, al-Sharif N, et al. Unilateral post-tuberculous lung destruction: the left bronchus syndrome. Thorax. 1990. 45:210–212.19. Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006. 61:259–266.20. Jordan TS, Spencer EM, Davies P. Tuberculosis, bronchiectasis and chronic airflow obstruction. Respirology. 2010. 15:623–628.21. Cleverley JR, Müller NL. Advances in radiologic assessment of chronic obstructive pulmonary disease. Clin Chest Med. 2000. 21:653–663.22. Madani A, Keyzer C, Gevenois PA. Quantitative computed tomography assessment of lung structure and function in pulmonary emphysema. Eur Respir J. 2001. 18:720–730.23. Kinsella M, Müller NL, Abboud RT, Morrison NJ, DyBuncio A. Quantitation of emphysema by computed tomography using a "density mask" program and correlation with pulmonary function tests. Chest. 1990. 97:315–321.24. Pande JN, Jain BP, Gupta RG, Guleria JS. Pulmonary ventilation and gas exchange in bronchiectasis. Thorax. 1971. 26:727–733.25. Loubeyre P, Paret M, Revel D, Wiesendanger T, Brune J. Thin-section CT detection of emphysema associated with bronchiectasis and correlation with pulmonary function tests. Chest. 1996. 109:360–365.26. Roberts HR, Wells AU, Milne DG, Rubens MB, Kolbe J, Cole PJ, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax. 2000. 55:198–204.27. Kim YJ, Park JY, Won JH, Kim CH, Kang DS, Jung TH. Lung volumes and diffusing capacity in bronchiectasis: correlation with the findings of high resolutional CT. Tuberc Respir Dis. 1999. 46:489–499.28. Kim YJ, Jung CY, Shin HW, Lee BK. Biomass smoke induced bronchial anthracofibrosis: presenting features and clinical course. Respir Med. 2009. 103:757–765.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Health-related Quality of Life Measurement with St. George's Respiratory Questionnaire in Post-tuberculous Destroyed Lung
- Effect of Inhaled Tiotropium on Spirometric Parameters in Patients with Tuberculous Destroyed Lung
- Prognostic Factors Affecting Postoperative Morbidity and Mortality in Destroyed Lung
- Validity and Reliability of CAT and Dyspnea-12 in Bronchiectasis and Tuberculous Destroyed Lung
- DILD (diffuse infiltrative lung disease); Radiologic Diagnostic Approach According to High-Resolution CT Pattern