Tuberc Respir Dis.
2006 Oct;61(4):366-373.
Paraquat-Induced Apoptotic Cell Death in Lung Epithelial Cells
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Dankook University, Cheonan, Korea.
- 2Department of Internal Medicine, Konkuk University Hospital School of Medicine, Konkuk University, Seoul, Korea. kyleemd@kuh.ac.kr
Abstract
-
BACKGROUND: Paraquat is extremely toxic chemical material, which generates reactive oxygen species (ROS), causing multiple organ failure. In particular, paraquat leads to irreversible progressive pulmonary fibrosis. Exaggerated cell deaths exceeding the normal repair of type II pneumocytes leads to mesenchymal cells proliferation and fibrosis. This study examined the followings; i) whether or not paraquat induces cell death in lung epithelial cells; ii) whether or not paraquat-induced cell deaths are apoptosis or necrosis; and iii) the effects of N-acetylcysteine, dexamethasone, and bcl-2 on paraquat-induced cell deaths.
METHODS
A549 and BEAS-2B lung epithelial cell lines were used. The cell viability and apoptosis were evalluated using a MTT assay, Annexin V staining was monitored by fluorescence microscopy, The level of bcl-2 inhibition was examined by establishing stable A549 pcDNA3-bcl-2 cell lines throung the transfection of pcDNA3-bcl-2 with the mock.
RESULTS
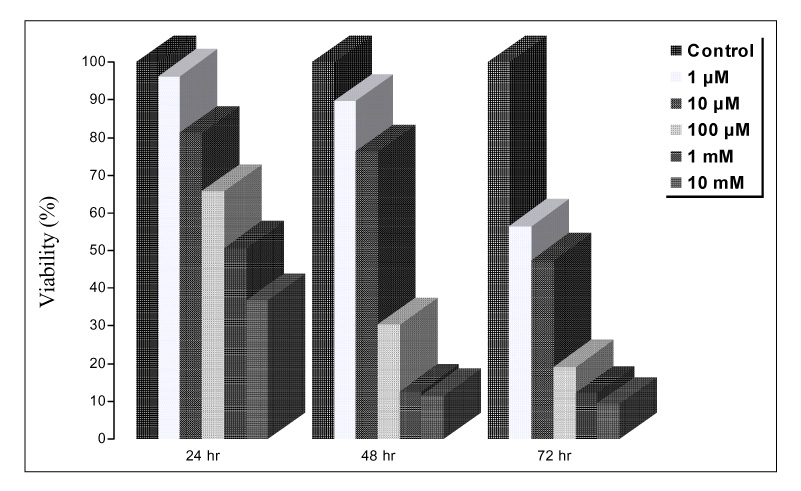
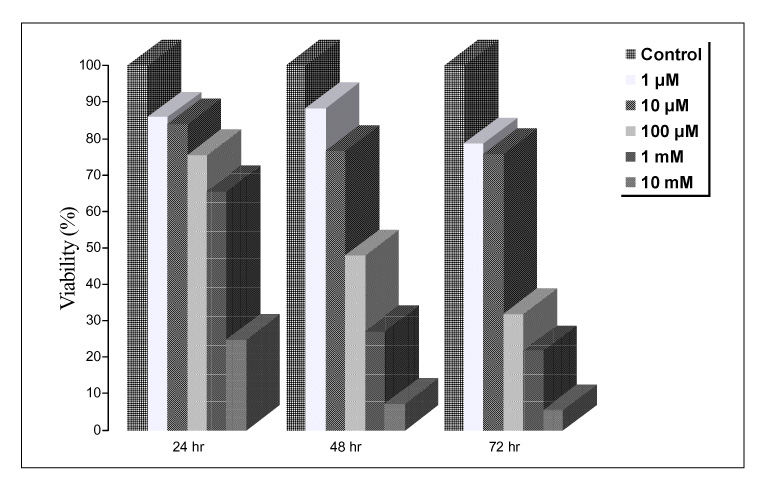
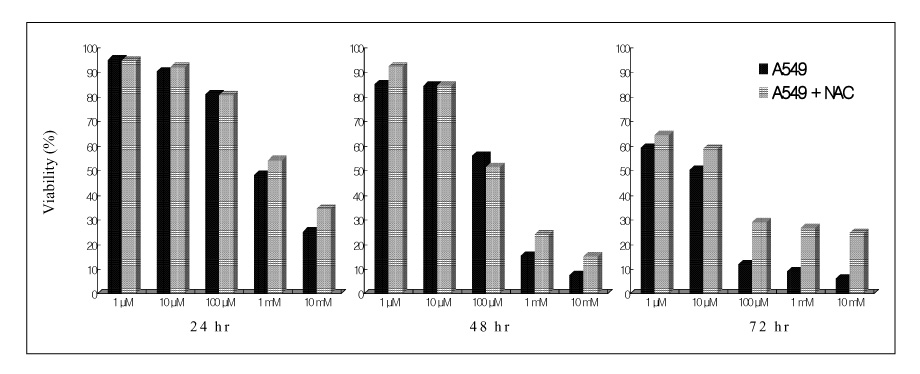
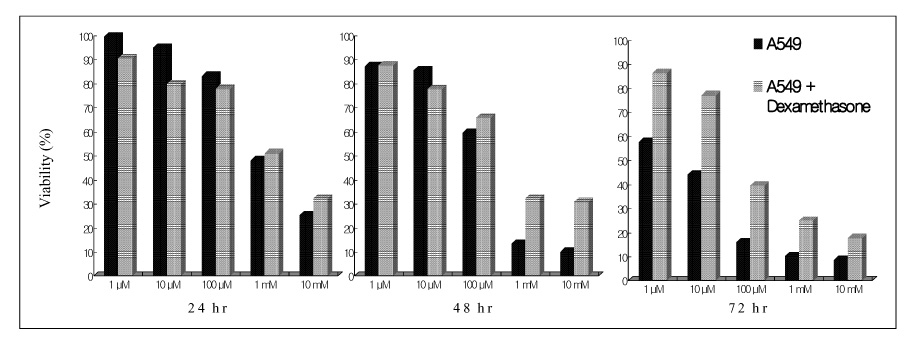
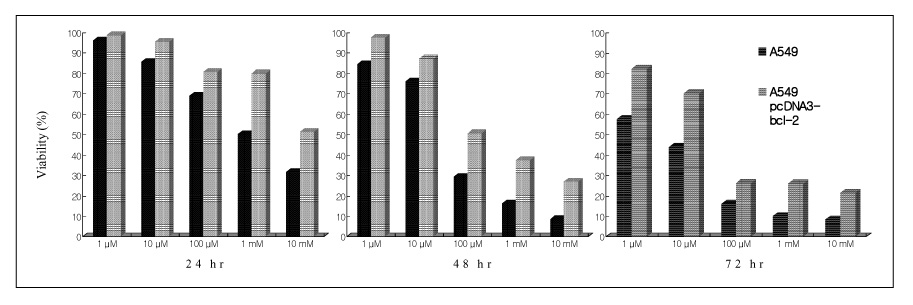
Paraquat decreased the cell viability in A549 and BEAS-2B cells in a dose and time dependent manner. The Annexin V assay showed that apoptosis was the type of paraquat-induced cell death. Paraquat-induced cell deaths was significantly inhibited by N-acetylcysteine, dexamethasone, and bcl-2 overexpression. The cell viability of A549 cells treated with N-acetylcysteine, and dexamethasone on the paraquat-induced cell deaths were increased significantly by 10 ~ 20%, particularly at high doses. In addition, the cell viability of A549 pcDNA3-bcl-2 cells overexpressing bcl-2 was significantly higher than the untransfected A549 cells.
CONCLUSION
Paraquat induces apoptotic cell deaths in lung epithelial cells in a dose and time dependent manner. The paraquat-induced apoptosis of lung epithelial cells might occur through the mitochondrial pathway.
Keyword
MeSH Terms
-
Acetylcysteine
Annexin A5
Apoptosis
Cell Death*
Cell Line
Cell Survival
Dexamethasone
Epithelial Cells*
Fibrosis
Lung*
Microscopy, Fluorescence
Multiple Organ Failure
Necrosis
Paraquat
Pneumocytes
Pulmonary Fibrosis
Reactive Oxygen Species
Transfection
Acetylcysteine
Annexin A5
Dexamethasone
Paraquat
Reactive Oxygen Species
Figure
Reference
-
1. Aldrich TK, Fisher AB, Cadenas E, Chance B. Evidence for lipid peroxidation by paraquat in the perfused rat lung. J Lab Clin Med. 1983. 101:66–73.2. Skillrud DM, Martin WJ 2nd. Paraquat-induced injury of type II alveolar cells. Am Rev Respir Dis. 1984. 129:995–999.3. Lee TH, Yang SW, LEE TG, Choi SY, Lee YW, Lee KY. Therapeutic effect of hemoperfusion in paraquat poisoning patients. Kor J Int Med. 1997. 52:114–119.4. Lee SI, Ahn KW, Chung CH. The effect of aminotriazole on pulmonaty toxicity of paraquat poisoning. Tuber Respir Dis. 1994. 41:222–230.5. Uhal BD, Joshi L, True AL, Mundle S, Raza A, Pardo A, et al. Fibroblasts isolated after fibrotic lung injury induce apoptosis of alveolar epithelial cells in vitro. Am J Physiol. 1995. 269:L819–L828.6. Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, et al. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharideinduced lung injury in mice. Am J Respir Crit Care Med. 2001. 163:762–769.7. Mo EK, Lee JH, Yoo CG, Kim YW, Han K, et al. The effect of TNF-α gene trasfer on chemosensitivity in lung cancer cells. Reports of Korean Tuberculosis Research Center. 1996. 7:29–54.8. Dusinska M, Kovacilova Z, Vallova B, Collins A. Responses of alveolar macrophages and epithelial type II cells to oxidative DNA damage caused by paraquat. Carcinogenesis. 1998. 19:809–812.9. Mossman BT, Gee JB. Pulmonary reactions and mechanisms of toxicity of inhaled fibers. Toxicol Appl Pharmacol. 1988. 93:472–483.10. Rose MS, Smith LL, Wyatt I. Evidence for energydependent accumulation of paraquat into rat lung. Nature. 1974. 252:314–315.11. Hagimoto N, Kuwano K, Nomoto Y, Kunitake R, Hara N. Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol. 1997. 16:91–101.12. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994. 149:818–824.13. Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995. 12:232–237.14. Woo D. Apoptosis and loss of renal tissue in polycystic kidney disease. N Engl J Med. 1995. 333:18–25.15. Hashimoto S, Kobayashi A, Kooguchi K, Kitamura Y, Onodera H, Nakajima H. Upregulation of two death pathways of perforin/granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000. 161:237–243.16. Matute G, Liles WC, Radella F, Steinberg KP, Ruzinski JT, Jonas M, et al. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997. 156:1969–1977.17. Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990. 142:1250–1257.18. Svee K, White J, Vaillant P, Jessurun J, Roongta U, Krumwlede M, et al. Acute lung injury fibroblast migration and invasion of a fibrin matrix is medigated by CD44. J Clin Invest. 1996. 98:1713–1727.19. Auphan N, Didonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995. 270:286–290.20. Lee KY, Kim YS, Ko MH, Park JS, Jee YK, Kim KY. Dexamethasone-induced inhibition of NF-κB transactivation in lung epithelial cells. Tuber Respir Dis. 2000. 48:682–698.21. Rahma I, Li XY, Donaldson K, Harrison DJ, MacNee W. Glutathione homeostasis in alveolar epithelial cells in vitro and lung in vivounder oxidative stress. Am J Physiol. 1995. 269:L285–L292.22. Bailly-Maitre B, Sousa G, Boulukos K, Gugenheim J, Rahmani R. Dexamethasone inhibits spontaneous apoptosis in primary cultures of human and rat hepatocytes via Bcl-2 and Bcl-xL induction. Cell Death Differ. 2001. 8:279–288.23. Fassina G, Giunciuglio D, Aluigi MG, Cai T, Masiello L, Dagostino F, et al. Inhibition of angiogenesis and protection from apoptosis by N-acetylcysteine. Cancer Detect Prev. 1998. 22:125–126.24. Hoffer E, Avidor I, Benjaminov O, Shenker L, Tabak A, Tamir A, et al. N-acetylcysteine delays the infiltration of inflammatory cells into the lungs of paraquat intoxicated rats. Toxicol Appl Pharmacol. 1993. 120:8–12.25. Hoffer E, Baum Y, Tabak A, Taitelman U. N-acetylcysteine increases the glutathione content and protects rat alveolar type II cells against paraquatinduced cytotoxicity. Toxicol Lett. 1996. 84:7–12.26. Fabisiak JP, Kagan VE, Ritov VB, Jonhson DE, Lazo JS. Bcl-2 inhibits selective oxidation and externalization of phosphatidylserine during paraquat-induced apoptosis. Am J Physiol. 1997. 272:C675–C684.27. Rimpler MM, Rauen U, Schmidt T, Moroy T, de Groot H. Protection against hydrogen peroxide cytotoxicity in Rat-1 fibroblasts provided by the oncoprotein Bcl-2: maintenance of calciumhomeostasis is secondary to the effect of Bcl-2 on cellular glutathione. Biochem J. 1999. 340:291–297.28. Guinee D Jr, Brambilla E, Fleming M, Hayashi T, Rahn M, Koss M, et al. The potential role of Bax and Bcl-2 expression in diffuse alveolar damage. Am J Pathol. 1997. 151:999–1007.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptosis in Respiratory Epithelial Cells: Triggering by Paraquat and Modulation by L-ascorbic acid
- Characterization of Nitric Oxide (NO)-Induced Cell Death in Lung Epithelial Cells
- The Effects of High Glucose on Paraquat-induced Cell Injury in Human Retinal Pigment Epithelial Cells
- Effects of corticosteroid on the paraquat induced lung injury
- The Influence of Hypo-Oxygenation or Hyper-Oxygenation on Cell Survival after Paraquat Exposure in A549 Cells