Prog Med Phys.
2013 Mar;24(1):25-34.
Voxel-based Investigations of Phase Mask Effects on Susceptibility Weighted Images
- Affiliations
-
- 1Department of Radiology, Kyung Hee University Hospital at Gangdong, School of Medicine, Kyung Hee University, Seoul, Korea. ghjahng@gmail.com
- 2Department of Biomedical Engineering, College of Electronic Information Engineering, Kyung Hee University, Yongin, Korea.
Abstract
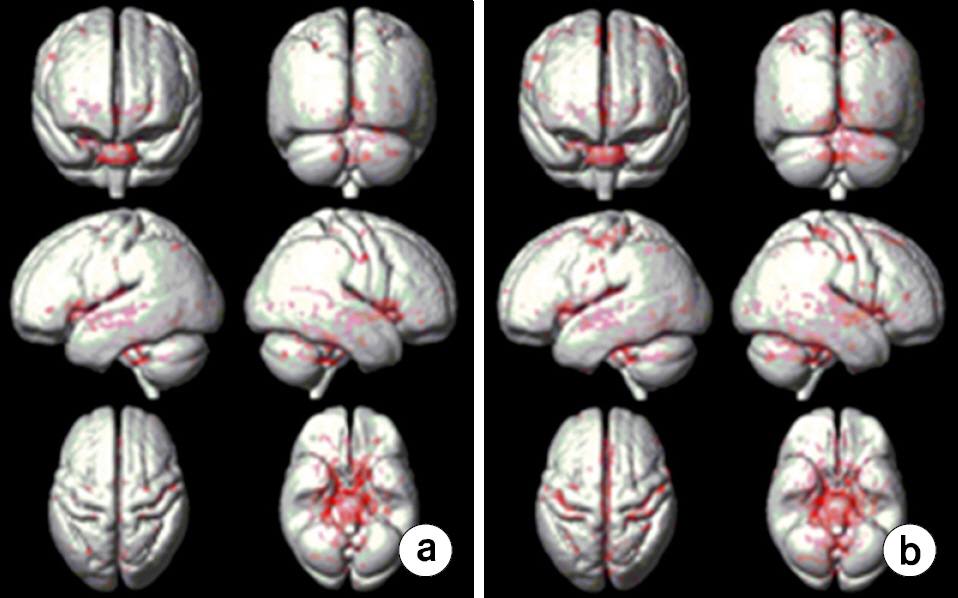
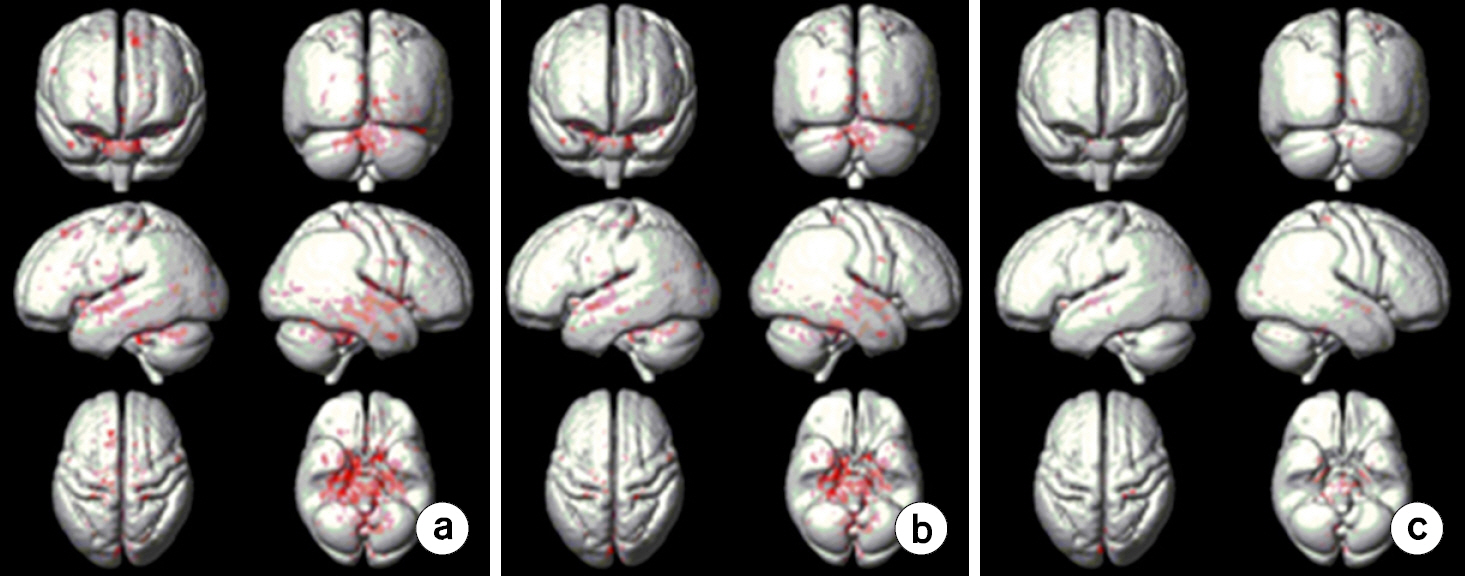
- To investigate effects of phase mask on susceptibility-weighted images (SWI) using voxel-based analyses in normal elderly subjects. A three-dimensional (3D) gradient echo sequence ran to obtain SWIs in 20 healthy elderly subjects. SWIs with two (SWI2) and four (SWI4) phase multiplications were achieved with positive (PSWI) and negative (NSWI) phase masks to investigate phase mask effects. The voxel-based comparisons were performed using paired t-tests between PSWI and NSWI and between SWI2 and SWI4. Differences of signal intensities between magnitude images and SWI4 were larger than those between magnitude images and SWI2s. Differences of signal intensities between magnitude images and PSWIs were larger than those between magnitude images and NSWIs. Moreover, the signal intensities from NSWI2s and NSWI4s were greater than those from PSWI2s and PSWI4s, respectively. More differences of signal intensities between NSWI4 and PSWI4s were found than those between NSWI2s and PSWI2s in the whole brain images. The voxel-based analyses of SWI could be beneficial to investigate susceptibility differences on the entire brain areas. The phase masking method could be chosen to enhance brain tissue contrast rather than to enhance venous blood vessels. Therefore, it is recommended to apply voxel-based analyses of SWI to investigate clinical applications.
MeSH Terms
Figure
Reference
-
References
1. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 52(3):612–618. 2004.
Article2. Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 30(1):19–30. 2009.
Article3. Rauscher A, Sedlacik J, Barth M, Mentzel HJ, Reichenbach JR. Magnetic susceptibility-weighted MR phase imaging of the human brain. AJNR Am J Neuroradiol. 26(4):736–742. 2005.4. Haacke EM, Cheng NY, House MJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 23(1):1–25. 2005.
Article5. Xu X, Wang Q, Zhang M. Age, gender, and hemispheric differences in iron deposition in the human brain: an in vivo MRI study. Neuroimage. 40(1):35–42. 2008.
Article6. Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. MRI estimates of brain iron concentration in normal aging: comparison of field-dependent (FDRI) and phase (SWI) methods. Neuroimage. 47(2):493–500. 2009.
Article7. Haacke EM, Miao Y, Liu M, et al. Correlation of putative iron content as represented by changes in R2∗ and phase with age in deep gray matter of healthy adults. J Magn Reson Imaging. 32(3):561–576. 2010.
Article8. Kim MJ, Jahng GH, Lee HY, et al. Development of a Korean standard structural brain template in cognitive normals and patients with mild cognitive impairment and Alzheimer's disease. J Korean Soc Magn Reson Med. 14(2):103–114. 2010.
Article9. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 19(3):1233–1239. 2003.
Article10. Eissa A, Lebel RM, Korzan JR, Catz I, et al. Detecting lesions in multiple sclerosis at 4.7 tesla using phase susceptibility-weighting and T2-weighting. J Magn Reson Imaging. 30(4):737–742. 2009.
Article11. Grabner G, Dal-Bianco A, Schernthaner M, Vass K, Lassmann H, Trattnig S. Analysis of multiple sclerosis lesions using a fusion of 3.0 T FLAIR and 7.0 T SWI phase: FLAIR SWI. J Magn Reson Imaging. 33(3):543–549. 2011.
Article12. Chamberlain R, Reyes D, Curran GL, et al. Comparison of amyloid plaque contrast generated by T2-weighted, T2∗-weighted, and susceptibility-weighted imaging methods in transgenic mouse models of Alzheimer's disease. Magn Reson Med. 61(5):1158–1164. 2009.13. Niwa T, Aida N, Kawaguchi H, et al. Anatomic dependency of phase shifts in the cerebral venous system of neonates at susceptibility-weighted MRI. J Magn Reson Imaging. 34(5):1031–1036. 2011.
Article14. Shmueli K, de Zwart JA, van Gelderen P, Li TQ, Dodd SJ, Duyn JH. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med. 62(6):1510–1522. 2009.
Article15. Schafer A, Wharton S, Gowland P, Bowtell R. Using magnetic field simulation to study susceptibility-related phase contrast in gradient echo MRI. Neuroimage. 48(1):126–137. 2009.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gaussian Filtering Effects on Brain Tissue-masked Susceptibility Weighted Images to Optimize Voxel-based Analysis
- Clinical Applications of Neuroimaging with Susceptibility Weighted Imaging: Review Article
- Effect of Voxel Size on the Accuracy of Landmark Identification in Cone-Beam Computed Tomography Images
- Findings Regarding an Intracranial Hemorrhage on the Phase Image of a Susceptibility-Weighted Image (SWI), According to the Stage, Location, and Size
- Factors affecting modulation transfer function measurements in cone-beam computed tomographic images