Korean J Urol.
2011 Jan;52(1):55-63.
Sunitinib Malate Synergistically Potentiates Anti-Tumor Effect of Gemcitabine in Human Bladder Cancer Cells
- Affiliations
-
- 1Department of Urology, Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. selee@snubh.org
Abstract
- PURPOSE
Sunitinib malate (Sutent; Pfizer, New York, NY, USA) is a highly selective multi-targeted agent and has been reported to have potent anti-tumor effects against various tumors, including renal cell carcinoma and gastrointestinal stromal tumors. In this study, we explored in vitro the anti-tumor effect and related molecular mechanisms of sunitinib malate against human bladder cancer cell lines. We also determined the synergistic anti-tumor effect between sunitinib and conventional cytotoxic drugs, cisplatin and gemcitabine, in bladder cancer cells.
MATERIALS AND METHODS
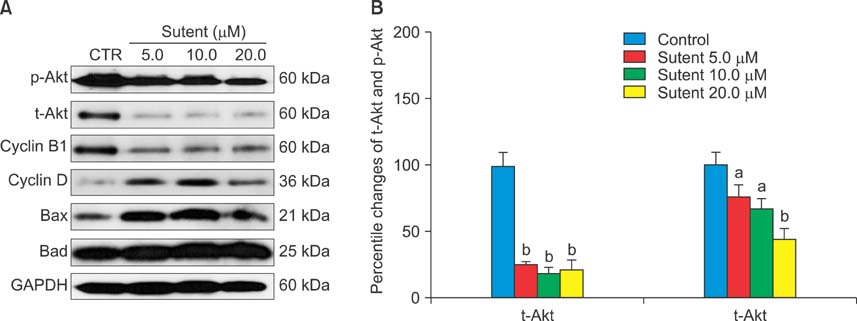
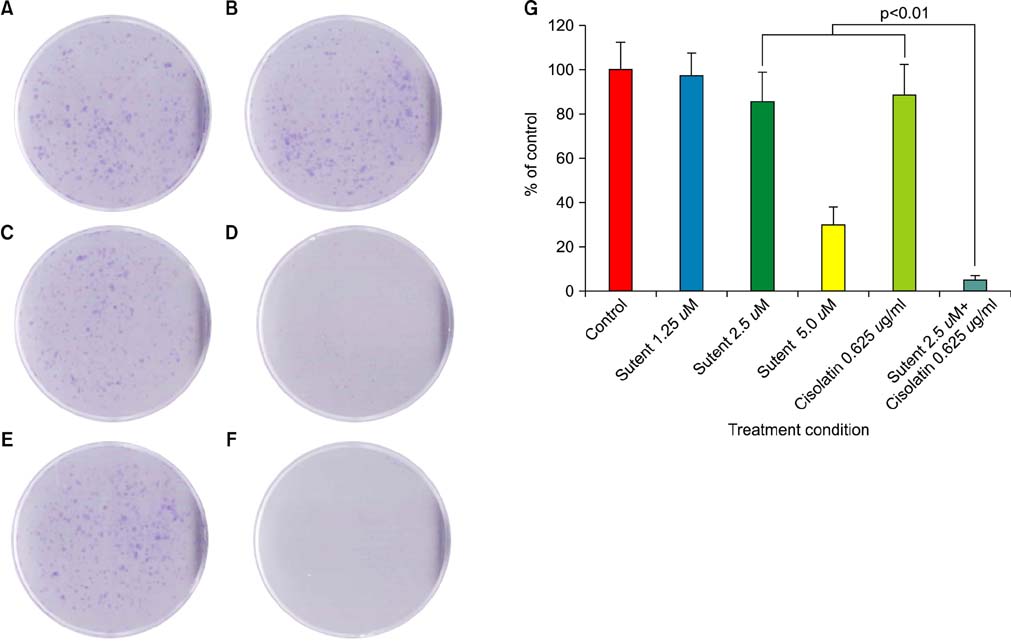
Six human cancer cell lines (HTB5, HTB9, T24, UMUC14, SW1710, and J82) were exposed to an escalating dose of sunitinib alone or in combination with cisplatin/gemcitabine, and the cytotoxic effect of the drugs was examined by CCK-8 assay. The synergistic effect between sunitinib and cisplatin/gemcitabine was determined by the combination index (CI) and clonogenic assay. Alterations in cell cycle (cyclin D, B1), survival (p-Akt, t-Akt), and apoptosis (Bax, Bad) regulator expression were analyzed by Western blotting.
RESULTS
Like cisplatin and gemcitabine, sunitinib exerted a dose- and time-dependent anti-tumor effect in bladder cancer cells. However, sunitinib exhibited entirely different sensitivity profiles from cisplatin and gemcitabine. Sunitinib suppressed the expression of cyclin B1, p-Akt, and t-Akt while augmenting the expression of cyclin D and pro-apoptotic Bax and Bad in HTB5 cells. Analysis of the drug combination by the isobolic method and clonogenic assay revealed that sunitinib acts in synergy with gemcitabine in HTB5 cells.
CONCLUSIONS
These results indicate that sunitinib malate has a potent anti-tumor effect and may synergistically enhance the anti-tumor effect of gemcitabine in human bladder cancer cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Fossa SD, Aass N, Ous S, Waehre H, Ilner K, Hannisdal E. Survival after curative treatment of muscle-invasive bladder cancer. Acta Oncol. 1996. 35:Suppl 8. 59–65.2. von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005. 23:4602–4608.3. Witte RS, Elson P, Bono B, Knop R, Richardson RR, Dreicer R, et al. Eastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. J Clin Oncol. 1997. 15:589–593.4. Sweeney CJ, Roth BJ, Kabbinavar FF, Vaughn DJ, Arning M, Curiel RE, et al. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol. 2006. 24:3451–3457.5. Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, et al. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007. 12:107–113.6. Roskoski R Jr. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun. 2007. 356:323–328.7. Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006. 295:2516–2524.8. Saltz LB, Rosen LS, Marshall JL, Belt RJ, Hurwitz HI, Eckhardt SG, et al. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol. 2007. 25:4793–4799.9. Socinski MA, Novello S, Brahmer JR, Rosell R, Sanchez JM, Belani CP, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008. 26:650–656.10. Bernardini S, Fauconnet S, Chabannes E, Henry PC, Adessi G, Bittard H. Serum levels of vascular endothelial growth factor as a prognostic factor in bladder cancer. J Urol. 2001. 166:1275–1279.11. Theodoropoulos VE, Lazaris AC, Kastriotis I, Spiliadi C, Theodoropoulos GE, Tsoukala V, et al. Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU Int. 2005. 95:425–431.12. Theodoropoulos VE, Lazaris A, Sofras F, Gerzelis I, Tsoukala V, Ghikonti I, et al. Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol. 2004. 46:200–208.13. Xia G, Kumar SR, Hawes D, Cai J, Hassanieh L, Groshen S, et al. Expression and significance of vascular endothelial growth factor receptor 2 in bladder cancer. J Urol. 2006. 175:1245–1252.14. Wu W, Shu X, Hovsepyan H, Mosteller RD, Broek D. VEGF receptor expression and signaling in human bladder tumors. Oncogene. 2003. 22:3361–3370.15. Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984. 22:27–55.16. Droller MJ. Vascular endothelial growth factor is a predictor of relapse and stage progression in superficial bladder cancer. J Urol. 1998. 160:1932.17. Crew JP, O'Brien T, Bradburn M, Fuggle S, Bicknell R, Cranston D, et al. Vascular endothelial growth factor is a predictor of relapse and stage progression in superficial bladder cancer. Cancer Res. 1997. 57:5281–5285.18. Chabannes E, Bernardini S, Wallerand H, Bittard H. Angiogenesis in bladder: prognosis indicator and therapeutic target. Prog Urol. 2001. 11:417–427.19. Szarvas T, Jäger T, Droste F, Becker M, Kovalszky I, Romics I, et al. Serum levels of angiogenic factors and their prognostic relevance in bladder cancer. Pathol Oncol Res. 2009. 15:193–201.20. Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008. 26:1810–1816.21. Silay MS, Miroglu C. Sunitinib malate and sorafenib may be beneficial at the treatment of advanced bladder cancer due to their anti-angiogenic effects. Med Hypotheses. 2007. 69:892–895.22. Castillo-Avila W, Piulats JM, Garcia Del Muro X, Vidal A, Condom E, Casanovas O, et al. Sunitinib inhibits tumor growth and synergizes with cisplatin in orthotopic models of cisplatin-sensitive and cisplatin-resistant human testicular germ cell tumors. Clin Cancer Res. 2009. 15:3384–3395.23. Dai CL, Liang YJ, Wang YS, Tiwari AK, Yan YY, Wang F, et al. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer Lett. 2009. 279:74–83.24. Cuneo KC, Geng L, Fu A, Orton D, Hallahan DE, Chakravarthy AB. SU11248 (sunitinib) sensitizes pancreatic cancer to the cytotoxic effects of ionizing radiation. Int J Radiat Oncol Biol Phys. 2008. 71:873–879.25. Vogl UM, Berger W, Micksche M, Pirker C, Lamm W, Pichelmeyer O, et al. Synergistic effect of Sorafenib and Sunitinib with Enzastaurin, a selective protein kinase C inhibitor in renal cell carcinoma cell lines. Cancer Lett. 2009. 277:218–226.26. Tran Cao HS, Bouvet M, Kaushal S, Keleman A, Romney E, Kim G, et al. Metronomic gemcitabine in combination with sunitinib inhibits multisite metastasis and increases survival in an orthotopic model of pancreatic cancer. Mol Cancer Ther. 2010. 9:2068–2078.27. Reck M, Frickhofen N, Cedres S, Gatzemeier U, Heigener D, Fuhr HG, et al. Sunitinib in combination with gemcitabine plus cisplatin for advanced non-small cell lung cancer: a phase I dose-escalation study. Lung Cancer. 2010. 70:180–187.28. Bharthuar A, Pearce L, Litwin A, LeVea C, Kuvshinoff B, Iyer R. Metastatic pancreatic adenocarcinoma and renal cell carcinoma treated with gemcitabine and sunitinib malate. A case report. JOP. 2009. 10:523–527.29. Sonpavde G, Jian W, Liu H, Wu MF, Shen SS, Lerner SP. Sunitinib malate is active against human urothelial carcinoma and enhances the activity of cisplatin in a preclinical model. Urol Oncol. 2009. 27:391–399.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Patient with Malignant Granular Cell Tumor Treated with Sunitinib Malate
- Effective Antiviral Activity of the Tyrosine Kinase Inhibitor Sunitinib Malate against Zika Virus
- Sunitinib-induced hypothyroidism
- Initial Experiences of Intravesical Gemcitabine Instillation Followed by Bacillus Calmette-Guerin(BCG) Therapy for Treating Intermediate or High Risk Patients with Superficial Bladder Cancer
- Sunitinib-induced hypothyroidism