Korean J Urol.
2011 Jul;52(7):466-473.
Role of the mTOR Pathway in the Progression and Recurrence of Bladder Cancer: An Immunohistochemical Tissue Microarray Study
- Affiliations
-
- 1Department of Urology, Chung-Ang University College of Medicine, Seoul, Korea. caucih@cau.ac.kr
- 2Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Numerous trials have been conducted to develop new treatment regimens for superficial and invasive bladder cancer, because there is an urgent need to identify novel agents to prevent the recurrence and progression of these cancers. We evaluated the prognostic and biological significance of mTOR pathway-related markers in patients with bladder cancer who had undergone transurethral resection of their bladder tumors and radical cystectomy.
MATERIALS AND METHODS
We retrieved 208 bladder cancer specimens collected from patients between 1989 and 2007 and constructed a tissue microarray comprising 208 tumor samples and 25 benign urothelium samples. Immunohistochemical staining was performed for mTOR, phosphorylated (phos) S6, and phos4E-BP1. The pattern, percentage, and intensity of staining for all three markers were evaluated.
RESULTS
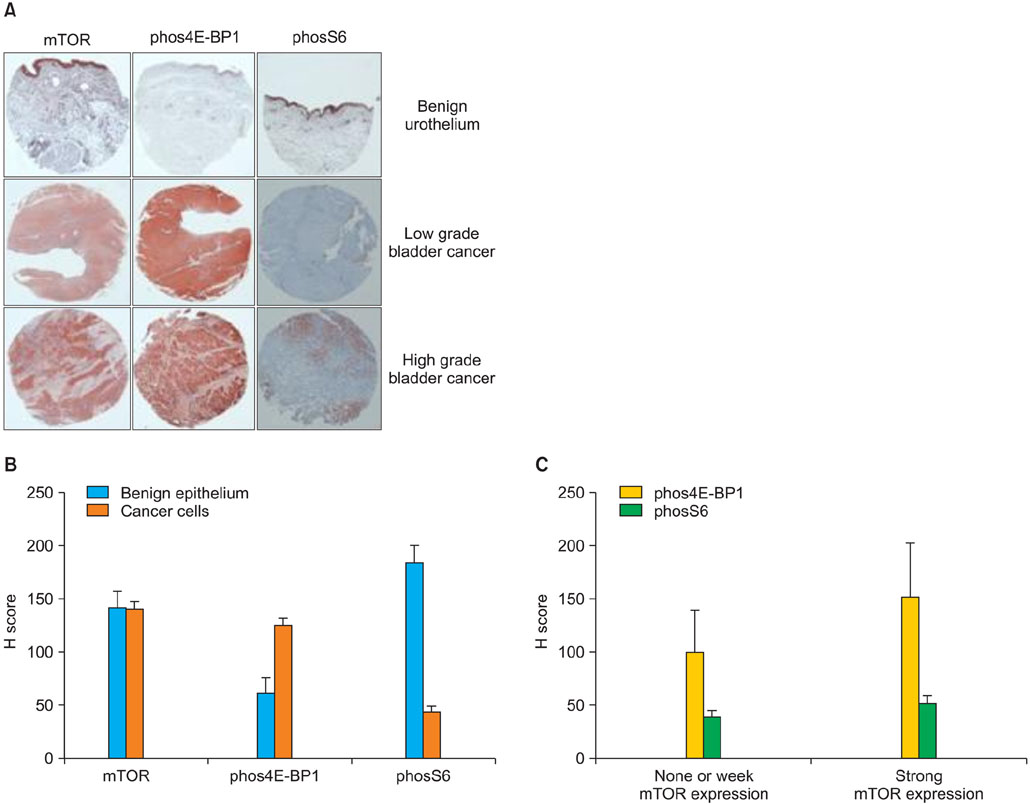
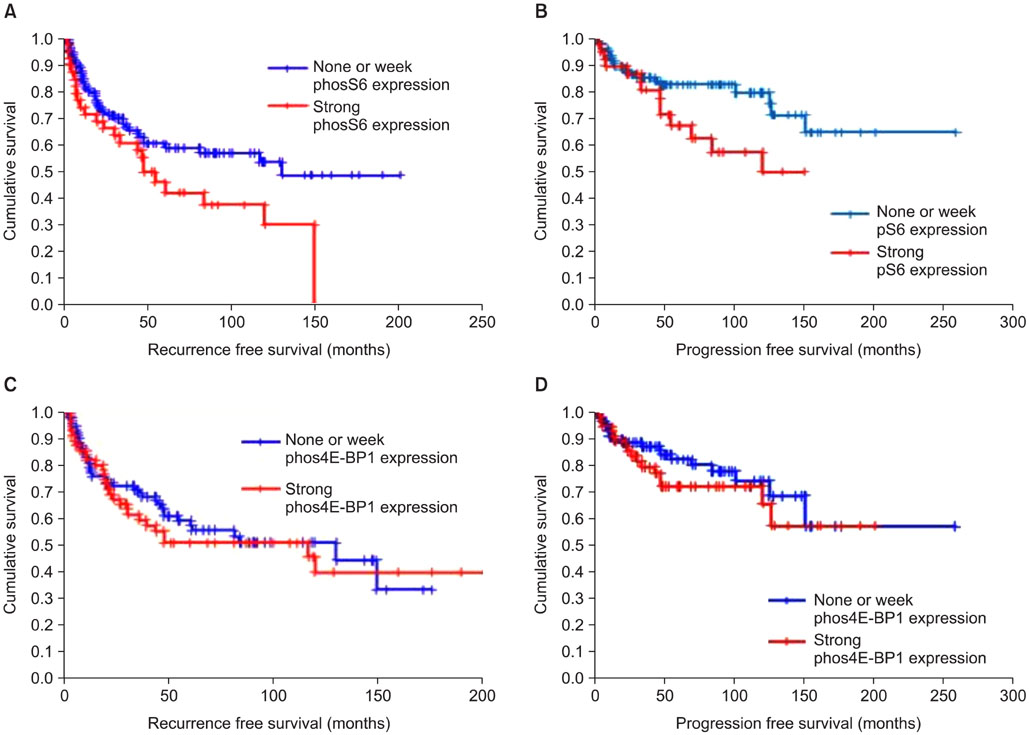
The median age at diagnosis of the patient cohort was 67 years (range: 29-87 years), and the median follow-up was 72 months (range: 1-257 months). The expression of phos4E-BP1 was higher in the bladder cancer cohort than in the benign cohort, whereas phosS6 expression was lower in the bladder cancer cohort than in the benign cohort. The expression of phosS6 was significantly higher in high-grade bladder cancer (p<0.01). There was a significant positive correlation between the H-scores of mTOR and phos4E-BP1 (coefficient of correlation, r=0.37, p<0.01) as well as between the H-scores of mTOR and phosS6 (r=0.17, p<0.05). In the multivariate analysis, strong phosS6 expression predicted shorter progression (p<0.01; hazard ratio [HR], 2.516) and disease-specific survival (p<0.01; HR, 2.396) but not overall survival (p=0.112), whereas strong phos4E-BP1 expression was a predictor of disease-specific survival (p<0.05; HR, 2.105). Moreover, strong phosS6 expression predicted shorter recurrence-free (p<0.05) and progression-free (p<0.05) survival in the superficial bladder cancer cohort.
CONCLUSIONS
Our results demonstrate that mTOR pathway activation, as assessed by phos4E-BP1 phosphorylation, is related to bladder cancer tumorigenesis and that S6 protein phosphorylation is associated with a high level of disease recurrence and progression and poor cancer-specific survival.
Keyword
MeSH Terms
Figure
Reference
-
1. Dalbagni G. The management of superficial bladder cancer. Nat Clin Pract Urol. 2007. 4:254–260.2. Jung SJ, Chang HS, Park CH, Kim CI, Kim BH. Effectiveness of an immediate mitomycin C instillation in patients with superficial bladder cancer receiving periodic mitomycin C instillation. Korean J Urol. 2011. 52:323–326.3. Choueiri TK, Raghavan D. Chemotherapy for muscle-invasive bladder cancer treated with definitive radiotherapy: persisting uncertainties. Nat Clin Pract Oncol. 2008. 5:444–454.4. Fossa SD, Aass N, Ous S, Waehre H, Ilner K, Hannisdal E. Survival after curative treatment of muscle-invasive bladder cancer. Acta Oncol. 1996. 35:Suppl 8. 59–65.5. Sternberg CN, Donat SM, Bellmunt J, Millikan RE, Stadler W, De Mulder P, et al. Chemotherapy for bladder cancer: treatment guidelines for neoadjuvant chemotherapy, bladder preservation, adjuvant chemotherapy, and metastatic cancer. Urology. 2007. 69:1 Suppl. 62–79.6. Garcia JA, Danielpour D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol Cancer Ther. 2008. 7:1347–1354.7. Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006. 6:729–734.8. Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005. 16:525–537.9. Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009. 27:2278–2287.10. Robb VA, Karbowniczek M, Klein-Szanto AJ, Henske EP. Activation of the mTOR signaling pathway in renal clear cell carcinoma. J Urol. 2007. 177:346–352.11. Fechner G, Classen K, Schmidt D, Hauser S, Müller SC. Rapamycin inhibits in vitro growth and release of angiogenetic factors in human bladder cancer. Urology. 2009. 73:665–668.12. Pinto-Leite R, Botelho P, Ribeiro E, Oliveira PA, Santos L. Effect of sirolimus on urinary bladder cancer T24 cell line. J Exp Clin Cancer Res. 2009. 28:3.13. Bergkvist A, Ljungqvist A, Moberger G. Classification of bladder tumours based on the cellular pattern. Preliminary report of a clinical-pathological study of 300 cases with a minimum follow-up of eight years. Acta Chir Scand. 1965. 130:371–378.14. Epstein JI, Amin MB, Reuter VR, Mostofi FK. Bladder Consensus Conference Committee. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Am J Surg Pathol. 1998. 22:1435–1448.15. Detre S, Saclani Jotti G, Dowsett M. A "quickscore" method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995. 48:876–878.16. Dinney CP, McConkey DJ, Millikan RE, Wu X, Bar-Eli M, Adam L, et al. Focus on bladder cancer. Cancer cell. 2004. 6:111–116.17. Zhang ZT, Pak J, Huang HY, Shapiro E, Sun TT, Pellicer A, et al. Role of Ha-ras activation in superficial papillary pathway of urothelial tumor formation. Oncogene. 2001. 20:1973–1980.18. Sibley K, Cuthbert-Heavens D, Knowles MA. Loss of heterozygosity at 4p16.3 and mutation of FGFR3 in transitional cell carcinoma. Oncogene. 2001. 20:686–691.19. Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009. 15:6008–6017.20. King JC, Xu J, Wongvipat J, Hieronymus H, Carver BS, Leung DH, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet. 2009. 41:524–526.21. Carver BS, Tran J, Gopalan A, Chen Z, Shaikh S, Carracedo A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009. 41:619–624.22. Pantuck AJ, Seligson DB, Klatte T, Yu H, Leppert JT, Moore L, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007. 109:2257–2267.23. Qian CN, Furge KA, Knol J, Huang D, Chen J, Dykema KJ, et al. Activation of the PI3K/AKT pathway induces urothelial carcinoma of the renal pelvis: identification in human tumors and confirmation in animal models. Cancer Res. 2009. 69:8256–8264.24. Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, Wang X, Shen TH, Matos T, et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009. 23:675–680.25. Schultz L, Albadine R, Hicks J, Jadallah S, DeMarzo AM, Chen YB, et al. Expression status and prognostic significance of mammalian target of rapamycin pathway members in urothelial carcinoma of urinary bladder after cystectomy. Cancer. 2010. 116:5517–5526.26. Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006. 25:6423–6435.27. Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990. 345:544–547.28. De Benedetti A, Harris AL. eIF4E expression in tumors: its possible role in progression of malignancies. Int J Biochem Cell Biol. 1999. 31:59–72.29. Baba HA, Wohlschlaeger J, Cicinnati VR, Hilgard P, Lang H, Sotiropoulos GC, et al. Phosphorylation of p70S6 kinase predicts overall survival in patients with clear margin-resected hepatocellular carcinoma. Liver Int. 2009. 29:399–405.30. Bu X, Jia F, Wang W, Guo X, Wu M, Wei L. Coupled down-regulation of mTOR and telomerase activity during fluorouracil-induced apoptosis of hepatocarcinoma cells. BMC Cancer. 2007. 7:208.31. Bansal K, Narayana Y, Patil SA, Balaji KN. M. bovis BCG induced expression of COX-2 involves nitric oxide-dependent and -independent signaling pathways. J Leukoc Biol. 2009. 85:804–816.32. Seager CM, Puzio-Kuter AM, Patel T, Jain S, Cordon-Cardo C, Mc Kiernan J, et al. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev Res (Phila). 2009. 2:1008–1014.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of Downstream Genes of the mTOR Pathway that Predict Recurrence and Progression in Non-Muscle Invasive High-Grade Urothelial Carcinoma of the Bladder
- Letter to the Editor: Bioinformatics Analysis in Downstream Genes of the mTOR Pathway to Predict Recurrence and Progression of Bladder Cancer
- The Author's Response: Bioinformatics Analysis in Downstream Genes of the mTOR Pathway to Predict Recurrence and Progression of Bladder Cancer
- NBR1 and KIF14 Downstream of the Mammarian Target of Rapamycin Pathway Predict Recurrence in Nonmuscle Invasive Low Grade Urothelial Carcinoma of the Bladder
- Autophagy and urothelial carcinoma of the bladder: A review