Pediatr Allergy Respir Dis.
2011 Jun;21(2):115-122.
CD30 Activation Induced Eosinophil Apoptosis is Mediated by Caspase-9
- Affiliations
-
- 1Department of Pediatrics, The Catholic University of Korea School of Medicine, Seoul, Korea. jintackk@catholic.ac.kr
- 2Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Although CD30 is known to be expressed more on eosinophils undergoing apoptosis, it is still not known how CD30 activation leads to eosinophil apoptosis. In this study, we evaluated whether ligation of CD30 incites apoptosis and investigated whether the mechanisms of CD30 induced eosinophil apoptosis are dependent on caspase activation.
METHODS
We drew 90 mL of peripheral blood from healthy donors and then purified eosinophils using a MACS system. Expression of CD30 on eosinophils was measured, and eosinophils were cultured in wells pretreated with anti-CD30 mAb, isotype control immunoglobulin G1, interleukin (IL)-5, and dexamethasone in Roswell Park Memorial Institute 1640 media supplemented with 10% fetal bovine serum. Their rates of apoptosis were then compared using flow cytometry. To evaluate whether caspase-9 is involved in CD30-induced eosinophil apoptosis, the apoptotic rate was evaluated after the addition of caspase-9 inhibitor. The expression of procaspase-9 was also measured using Western blot.
RESULTS
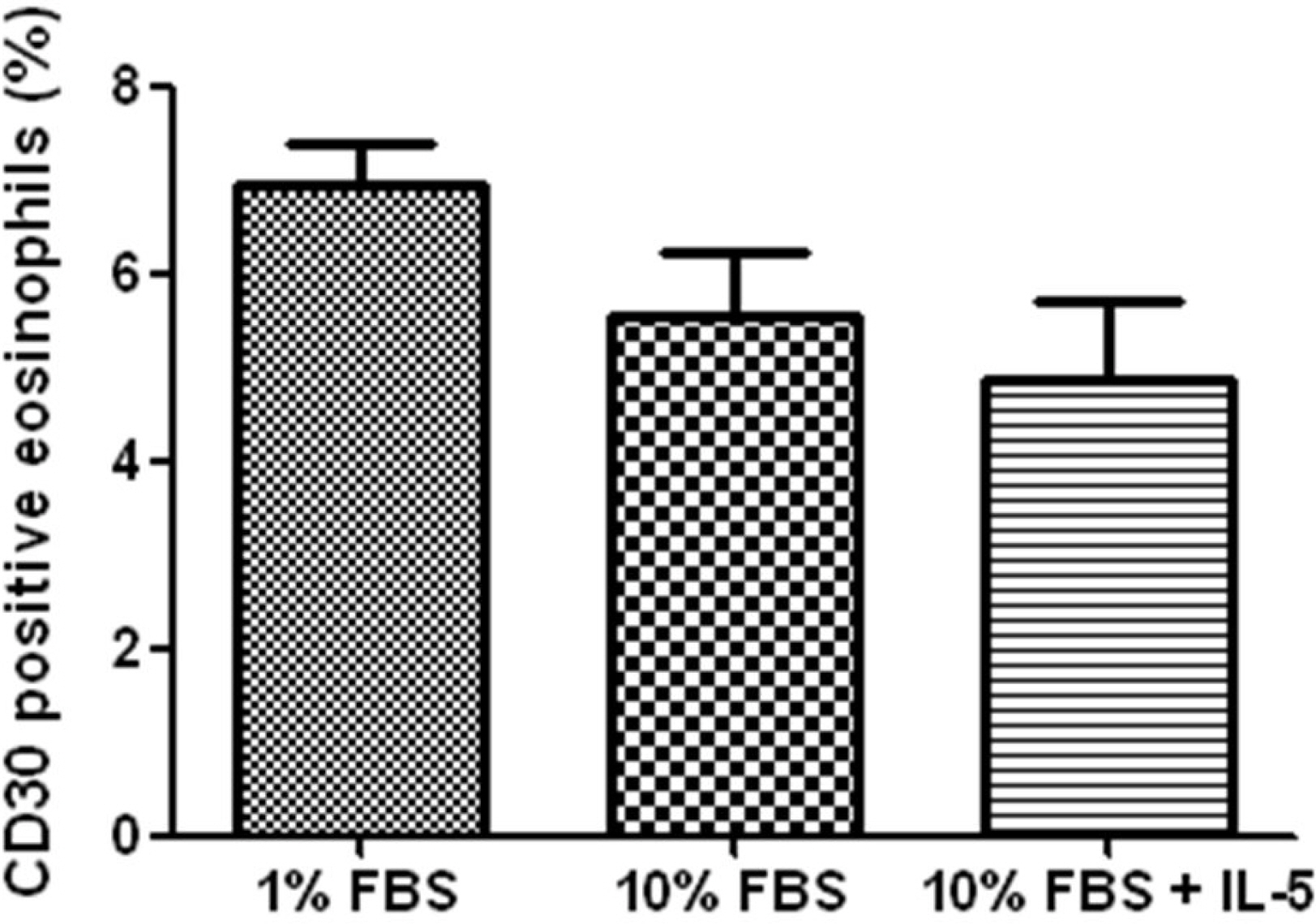
Expression of CD30 molecules on eosinophils increased steadily as the culture time lapse. The apoptotic rates of eosinophils cultured in the presence of anti-CD30 mAb were significantly increased to 29.1+/-6.1% and 47.3+/-4.7% compared to 17.1+/-6.7% and 29.4+/-9.2% of the control at 4 and 24 hours, respectively (both P<0.05). The apoptotic rates of eosinophils treated with anti-CD30 mAb were even faster than those of eosinophils treated with dexamethasone, and the mAb also suppressed the IL-5-induced enhancing effect of eosinophil survival. Caspase-9 inhibitor suppressed mAb induced eosinophil apoptosis from 54.8+/-6.9% and 71.5+/-11.6% to 24.5+/-6.0% and 47.8+/-11.4% at 18 and 36 hours, respectively (both P<0.001). We also demonstrated that the expression of procaspase-9 with mAb was diminished compared to that of the control and of IL-5.
CONCLUSION
This study showed CD30 activation enhances eosinophil apoptosis, and the effect is mediated by caspase-9 activation.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Gleich GJ. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990; 85:422–36.
Article2. Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000; 105:651–63.
Article3. Sanderson CJ. Interleukin-5: an eosinophil growth and activation factor. Dev Biol Stand. 1988; 69:23–9.4. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972; 26:239–57.
Article5. Allen RT, Hunter WJ 3rd, Agrawal DK. Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Methods. 1997; 37:215–28.
Article6. Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, et al. Human ICE/CED-3 protease nomenclature. Cell. 1996; 87:171.
Article7. Matsumoto K, Schleimer RP, Saito H, Iikura Y, Bochner BS. Induction of apoptosis in human eosinophils by anti-Fas antibody treatment in vitro. Blood. 1995; 86:1437–43.
Article8. Walsh GM, Williamson ML, Symon FA, Willars GB, Wardlaw AJ. Ligation of CD69 induces apoptosis and cell death in human eosinophils cultured with granulocyte-macrophage colonystimulating factor. Blood. 1996; 87:2815–21.
Article9. Blaylock MG, Sexton DW, Walsh GM. Ligation of CD45 and the isoforms CD45RA and CD45RB accelerates the rate of constitutive apoptosis in human eosinophils. J Allergy Clin Immunol. 1999; 104:1244–50.
Article10. Dürkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992; 68:421–7.
Article11. Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985; 66:848–58.
Article12. Jung W, Krueger S, Renner C, Gause A, Sahin U, Trümper L, et al. Opposite effects of the CD30 ligand are not due to CD30 mutations: results from cDNA cloning and sequence comparison of the CD30 antigen from different sources. Mol Immunol. 1994; 31:1329–34.
Article13. Masuda M, Ishida C, Arai Y, Okamura T, Ohsawa M, Shimakage M, et al. Dual action of CD30 antigen: anti-CD30 antibody induced apoptosis and interleukin-8 secretion in Ki-1 lymphoma cells. Int J Hematol. 1998; 67:257–65.14. Mir SS, Richter BW, Duckett CS. Differential effects of CD30 activation in anaplastic large cell lymphoma and Hodgkin disease cells. Blood. 2000; 96:4307–12.
Article15. Gruss HJ, Boiani N, Williams DE, Armitage RJ, Smith CA, Goodwin RG. Pleiotropic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood. 1994; 83:2045–56.
Article16. Muta H, Boise LH, Fang L, Podack ER. CD30 signals integrate expression of cytotoxic effector molecules, lymphocyte trafficking signals, and signals for proliferation and apoptosis. J Immunol. 2000; 165:5105–11.
Article17. Pinto A, Aldinucci D, Gloghini A, Zagonel V, Degan M, Improta S, et al. Human eosinophils express functional CD30 ligand and stimulate proliferation of a Hodgkin's disease cell line. Blood. 1996; 88:3299–305.
Article18. Chiarle R, Podda A, Prolla G, Gong J, Thorbecke GJ, Inghirami G. CD30 in normal and neoplastic cells. Clin Immunol. 1999; 90:157–64.
Article19. Berro AI, Perry GA, Agrawal DK. Increased expression and activation of CD30 induce apoptosis in human blood eosinophils. J Immunol. 2004; 173:2174–83.
Article20. Matsumoto K, Terakawa M, Miura K, Fukuda S, Nakajima T, Saito H. Extremely rapid and intense induction of apoptosis in human eosinophils by anti-CD30 antibody treatment in vitro. J Immunol. 2004; 172:2186–93.
Article21. Hansel TT, De Vries IJ, Iff T, Rihs S, Wand-zilak M, Betz S, et al. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991; 145:105–10.
Article22. Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997; 158:3902–8.23. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyperresponsiveness, and the late asthmatic response. Lancet. 2000; 356:2144–8.24. Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003; 167:199–204.
Article25. Matsumoto K, Terakawa M, Fukuda S, Saito H. Rapid and strong induction of apoptosis in human eosinophils by anti-CD30 mAb-coated microspheres and phagocytosis by macrophages. Int Arch Allergy Immunol. 2007; 143(Suppl 1):60–7.
Article26. Gedrich RW, Gilfillan MC, Duckett CS, Van Dongen JL, Thompson CB. CD30 contains two binding sites with different specificities for members of the tumor necrosis factor receptor-associated factor family of signal transducing proteins. J Biol Chem. 1996; 271:12852–8.
Article27. Horie R, Aizawa S, Nagai M, Ito K, Higashihara M, Ishida T, et al. A novel domain in the CD30 cytoplasmic tail mediates NFkappaB activation. Int Immunol. 1998; 10:203–10.
Article28. Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003; 14:193–209.
Article29. Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem. 1998; 273:2926–30.
Article30. Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997; 90:405–13.
Article31. Matsumoto K, Terakawa M, Fukuda S, Saito H. Analysis of signal transduction pathways involved in anti-CD30 mAb-induced human eosinophil apoptosis. Int Arch Allergy Immunol. 2010; 152(Suppl 1):2–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Caspases Activation in Ultraviolet B-induced Apoptosis of G361 Human Melanoma Cell Line
- Control of Mitochondrial Dynamics by Fas-induced Caspase-8 Activation in Hippocampal Neurons
- Induction and Regulation of CD30 Expression on Murine B Lymphocytes by Non-specific Stimulation
- Caspase-3 Activation Leads to Apoptosis of Human Gastric Epithelial Cells Infected with Helicobacter pylori
- Activation of caspase-8 in 3-deazaadenosine-induced apoptosis of U-937 cells occurs downstream of caspase-3 and caspase-9 without Fas receptor-ligand interaction