Pediatr Allergy Respir Dis.
2011 Mar;21(1):39-46.
Atopy as a Risk Factor for an Elevated Bronchodilator Response in Children with Asthma
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Seoul National University, Seoul, Korea. kohyy@plaza.snu.ac.kr
Abstract
- PURPOSE
The bronchodilator response (BDR) is frequently measured to assess the severity of asthma and to help facilitate therapeutic decisions, as well as to confirm the diagnosis. Few reports are available on the impact of atopy, one of the most important risk factors for childhood asthma, on the BDR.
METHODS
The medical records of 207 asthmatic children (174 with atopic asthma and 33 with non-atopic asthma) were retrospectively reviewed. At the time of asthma diagnosis, the subjects underwent blood tests, bronchial provocation tests, and spirometry before and 15 minutes after inhalation of 4 puffs of salbutamol. We compared the mean BDR levels between the children with atopic and non-atopic asthma, then determined the correlations between the BDR and serum markers of eosinophilic inflammation.
RESULTS
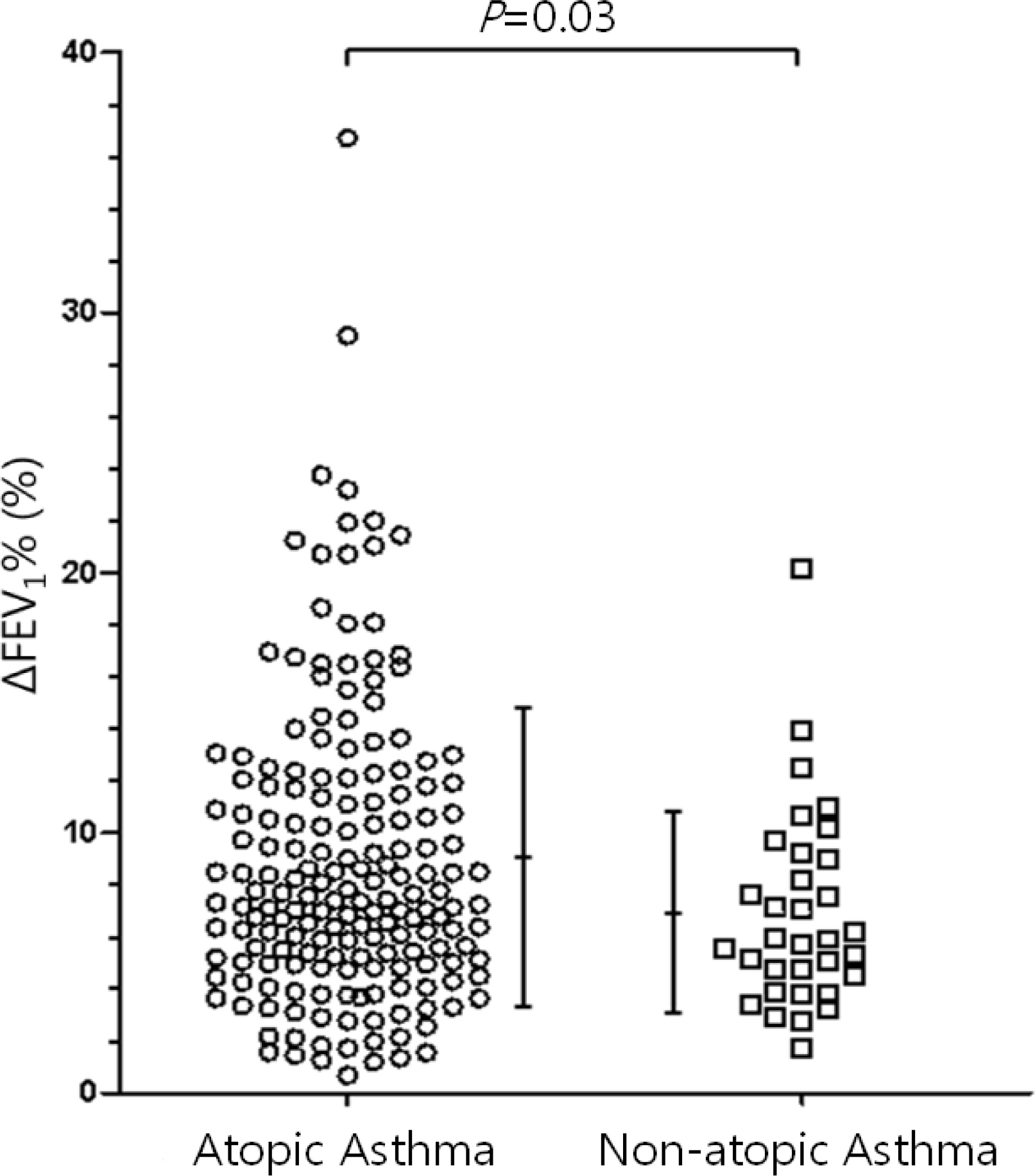
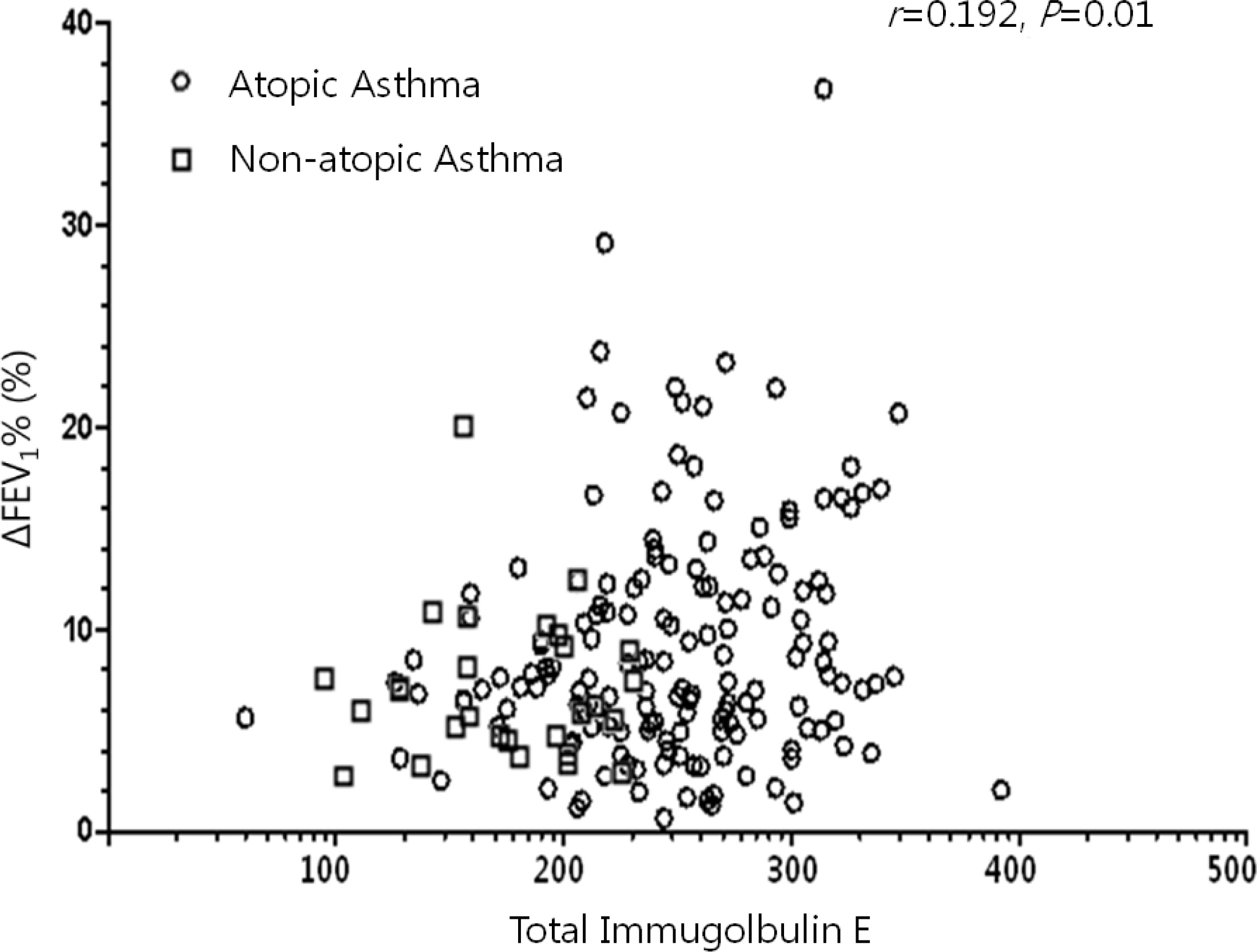
While the mean pre-bronchodilator FEV1 was not different between children with atopic and non-atopic asthma, atopic asthmatics had a higher mean BDR than non-atopic asthmatics (9.12+/-5.69% vs. 6.93+/-3.80%, P =0.03). There were weak, but significant correlations between the BDR and the serum markers of eosinophilic inflammation (total immunoglobulin E, r =0.192, P =0.01; total eosinophil count, r =0.192, P =0.01; eosinophil cationic protein, r =0.200, P <0.01).
CONCLUSION
Asthmatic children had different mean levels of BDR based on atopic status at the time of asthma diagnosis. When the BDR was assessed to aid therapeutic decisions, the presence of atopy should be taken into consideration in children with asthma.
Keyword
MeSH Terms
Figure
Reference
-
References
1. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007; 120(Suppl 1):94–138.2. Dales RE, Spitzer WO, Tousignant P, Schechter M, Suissa S. Clinical interpretation of airway response to a bronchodilator. Epidemiologic considerations. Am Rev Respir Dis. 1988; 138:317–20.
Article3. Tantisira KG, Fuhlbrigge AL, Tonascia J, Van Natta M, Zeiger RS, Strunk RC, et al. Bronchodilation and bronchoconstriction: Predictors of future lung function in childhood asthma. J Allergy Clin Immunol. 2006; 117:1264–71.
Article4. Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990; 323:1033–9.
Article5. Oosterhoff Y, Kauffman HF, Rutgers B, Zijlstra FJ, Koeter GH, Postma DS. Inflammatory cell number and mediators in bronchoalveolar lavage fluid and peripheral blood in subjects with asthma with increased nocturnal airways narrowing. J Allergy Clin Immunol. 1995; 96:219–29.
Article6. Takaku Y, Nakagome K, Kobayashi T, Yamaguchi T, Nishihara F, Soma T, et al. Changes in airway inflammation and hyperresponsiveness after inhaled corticosteroid cessation in allergic asthma. Int Arch Allergy Immunol;. 152(Suppl 1):41–6.
Article7. Abba AA. Exhaled nitric oxide in diagnosis and management of respiratory diseases. Ann Thorac Med. 2009; 4:173–81.
Article8. Niimi A, Amitani R, Suzuki K, Tanaka E, Murayama T, Kuze F. Serum eosinophil cationic pro-tein as a marker of eosinophilic inflammation in asthma. Clin Exp Allergy. 1998; 28:233–40.
Article9. Pumputiene I, Emuzyte R, Dubakiene R, Firan-tiene R, Tamosiunas V. T cell and eosinophil activation in mild and moderate atopic and nonatopic children's asthma in remission. Allergy. 2006; 61:43–8.
Article10. Demange V, Wild P, Zmirou-Navier D, Tossa P, Bohadana A, Barbaud A, et al. Associations of airway inflammation and responsiveness markers in non asthmatic subjects at start of apprenticeship. BMC Pulm Med. 2010; 37:1–10.
Article11. Mochizuki H, Shigeta M, Tokuyama K, Morikawa A. Difference in airway reactivity in children with atopic vs nonatopic asthma. Chest. 1999; 116:619–24.
Article12. Kelley CF, Mannino DM, Homa DM, Savage-Brown A, Holguin F. Asthma phenotypes, risk factors, and measures of severity in a national sample of US children. Pediatrics. 2005; 115:726–31.
Article13. Lee SH, Kim DK, Choi SH, Koh YY. Methacholine and adenosine 5'-monophosphate challenge tests in children with atopic asthma and with nonatopic asthma, and their relationships to blood eosinophil markers. Korean J Pediatr. 2006; 49:1216–22.
Article14. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2009 update. [cited 2010 November 27]; Available from:. http://www.ginasthma.com.15. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–38.16. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005; 26:948–68.17. Sub-Committee on skin tests of the European Academy of Allergology and Clinical Immunology. Skin tests used in type I allergy testing. Position paper. Allergy. 1989; 44(Suppl 10):1–59.18. Yoon KA, Lim HS, Koh YY, Kim H. Normal predicted values of pulmonary function test in Korean school-aged children. J Korean Pediatr Soc. 1993; 36:25–37.19. Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator respon-siveness in wheezy children. Thorax. 2005; 60:13–6.
Article20. Galant SP, Morphew T, Amaro S, Liao O. Value of the bronchodilator response in assessing controller naive asthmatic children. J Pediatr. 2007; 151:457–62.21. Suh DI, Lee JK, Lee JH, Koh YY. Bronchodilator Response and Its Relationship to Bronchial Hyperresponsiveness in Children with Allergic Rhinitis/Asthma. Pediatr Allergy Respir Dis (Korea). 2010; 20:59–67.22. Faul JL, Demers EA, Burke CM, Poulter LW. Alterations in airway inflammation and lung function during corticosteroid therapy for atopic asthma. Chest. 2002; 121:1414–20.
Article23. Folkerts G, Busse WW, Nijkamp FP, Sorkness R, Gern JE. Virus-induced airway hyperresponsiveness and asthma. Am J Respir Crit Care Med. 1998; 157:1708–20.
Article24. Cheung D, Dick EC, Timmers MC, de Klerk EP, Spaan WJ, Sterk PJ. Rhinovirus inhalation causes long-lasting excessive airway narrowing in response to methacholine in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1995; 152:1490–6.
Article25. Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989; 320:271–7.
Article26. Lindberg RE, Arroyave C. Levels of IgE in serum from normal children and allergic children as measured by an enzyme immunoassay. J Allergy Clin Immunol. 1986; 78:614–8.
Article27. Allen ND, Davis BE, Hurst TS, Cockcroft DW. Difference between dosimeter and tidal breathing methacholine challenge: contributions of dose and deep inspiration bronchoprotection. Chest. 2005; 128:4018–23.28. Chhabra SK, Vijayan VK, Gupta R, De S. Expression of bronchodilator response: comparison of four indices. Respir Med. 2002; 96:611–4.
Article29. Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild-to-moderate asthma. J Pediatr. 2003; 142:469–75.
Article30. Belda J, Leigh R, Parameswaran K, O'Byrne PM, Sears MR, Hargreave FE. Induced sputum cell counts in healthy adults. Am J Respir Crit Care Med. 2000; 161:475–8.
Article31. Travers J, Marsh S, Aldington S, Williams M, Shirtcliffe P, Pritchard A, et al. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J Respir Crit Care Med. 2007; 176:238–42.
Article32. Jang AS, Lee JH, Park SW, Park JS, Kim DJ, Park CS. Risk factors related to fixed airway obstruction in patients with asthma after antiasthma treatment. Ann Allergy Asthma Immunol. 2007; 99:408–12.
Article33. Apter AJ, Szefler SJ. Advances in adult and pediatric asthma. J Allergy Clin Immunol. 2006; 117:512–8.
Article34. Carroll WD, Jones PW, Boit P, Clayton S, Cliff I, Lenney W. Childhood evaluation of salmeterol tolerance-A double-blind randomized controlled trial. Pediatric Allergy Immunol. 2010; 21:336–44.35. Jang AS, Park JS, Lee JH, Park SW, Kim DJ, Uh ST, et al. Asthmatics without rhinitis have more fixed airway obstruction than those with concurrent rhinitis. Allergy Asthma Immunol Res. 2010; 2:108–13.
Article36. Zhong W, Levin L, Reponen T, Hershey GK, Adhikari A, Shukla R, et al. Analysis of short-term influences of ambient aeroallergens on pediatric asthma hospital visits. Sci Total Environ. 2006; 370:330–6.
Article37. Carpentiere G, Marino S, Castello F, Zarcone P, Cipolla G, Baldanza C. Oral sustained-release aminophylline and bronchodilator response to inhaled fenoterol in patients with chronic airflow obstruction. Chest. 1985; 88:576–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of bronchodilator response as an index of asthma control in children
- The role of inhaled and/or nasal corticosteroids on the bronchodilator response
- Bronchodilator Response and Its Relationship to Bronchial Hyperresponsiveness in Children with Allergic Rhinitis/Asthma
- A Clinical Study on Infantile Asthma
- Relationship Between Atopy and Bronchial Hyperresponsiveness