Korean J Urol.
2009 Oct;50(10):942-946.
Effectiveness of Computed Tomography for Predicting the Nuclear Grade of Renal Cell Carcinoma
- Affiliations
-
- 1Department of Urology, Keimyung University School of Medicine, Daegu, Korea. sangraal@dsmc.or.kr
Abstract
- PURPOSE
Nuclear grade is one of the independent prognostic factors for renal cell carcinoma (RCC). We investigated the effectiveness of a preoperative CT scan for predicting the nuclear grade of clear cell RCC.
MATERIALS AND METHODS
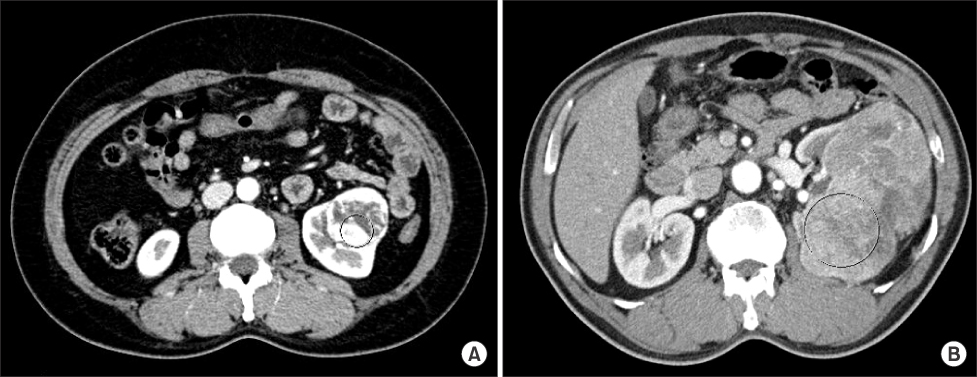
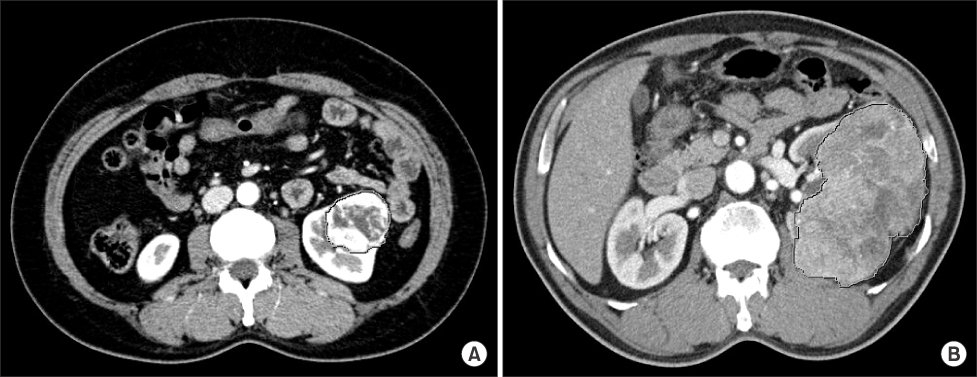
We retrospectively reviewed the medical records of patients who underwent surgery for renal lesions between January 2002 and December 2007. We analyzed the pathologic and radiologic reports of 65 patients who underwent radical nephrectomy for RCC and were diagnosed with clear cell RCC. The Hounsfield unit (HU) of the area with maximum enhancement (M) and the total area of the RCC (T) were measured during CT. Ratio values by nuclear grade were calculated by using formulas (M HU/aorta HU, T HU/aorta HU) to eliminate differences between individuals.
RESULTS
A total of 65 cases of clear cell RCCs were classified according to Fuhrman nuclear grade. Five cases were grade I, 33 were grade II, 15 were grade III, and 12 were grade IV. There was a significant difference in CT enhancement between each nuclear grade, and lower nuclear grades tended to have an increased ratio of maximum enhancement (p=0.020). Fuhrman nuclear grade was divided into two groups: low (Fuhrman grades I, II) and high (Fuhrman grades III, IV). The ratio of enhancement for the M area was significantly higher in the low Fuhrman nuclear grade group than in the high group (p=0.033).
CONCLUSIONS
CT enhancement is inversely related to the nuclear grade of clear cell RCC. This study found that measuring the area of maximum enhancement in CT may be a useful method for presuming the pathologic nuclear grade of RCC, especially when the Fuhrman nuclear grade is divided into low and high groups.
MeSH Terms
Figure
Reference
-
1. Lee HW, Cho KS, Jeong H, Yoon SJ, Jo MK, Lee ES, et al. Clinical analysis of incidentally found renal cell carcinoma: experiences of recent 8 years. Korean J Urol. 1998. 39:982–987.2. Seong BM, Kim DS, Yoon DK. Clinical characteristics of incidentally detected renal cell carcinoma. Korean J Urol. 1997. 38:245–249.3. Rhew HY, Kang JS, Jo SS, Lee CK. Clinical characteristics of incidentally detected renal cell carcinoma: incidentaloma. Korean J Urol. 2000. 41:1195–1201.4. Jamis-Dow CA, Choyke PL, Jennings SB, Linehan WM, Thakore KN, Walther MM. Small (< or = 3-cm) renal masses: detection with CT versus US and pathologic correlation. Radiology. 1996. 198:785–788.5. Campbell SC, Novick AC, Bukowsik RM. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Renal tumor. Campbell-Walsh urology. 2006. 9th ed. Philadelpia: Saunders;1603–1607.6. Minardi D, Lucarini G, Mazzucchelli R, Milanese G, Natali D, Galosi AB, et al. Prognostic role of Fuhrman grade and vascular endothelial growth factor in pT1a clear cell carcinoma in partial nephrectomy specimens. J Urol. 2005. 174:1208–1212.7. Boxer RJ, Waisman J, Lieber MM, Mampaso FM, Skinner DG. Renal carcinoma: computer analysis of 96 patients treated by nephrectomy. J Urol. 1979. 122:598–601.8. Villalobos-Gollas M, Leon-Vilchis F, Mendez-Probst C, Rodriguez-Covarrubias F, Castillejos-Molina R. Nuclear grade prediction of renal cell carcinoma using contrasted computed tomography. J Urol. 2009. 181:Suppl. 249.9. Boring CC, Squires TS, Tong T. Cancer statistics, 1993. CA Cancer J Clin. 1993. 43:7–26.10. Kim KJ, Kim DS, Lee NK. Prognosis associated with thrombocytosis in renal cell carcinoma. Korean J Urol. 2007. 48:1099–1103.11. Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003. 27:612–624.12. Tsui KH, Shvarts O, Smith RB, Figlin RA, deKernion JB, Belldegrun A. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000. 163:1090–1095.13. Ljungberg B, Alamdari FI, Holmberg G, Granfors T, Duchek M. Radical nephrectomy is still preferable in the treatment of localized renal cell carcinoma. A long-term follow-up study. Eur Urol. 1998. 33:79–85.14. Mejean A, Oudard S, Thiounn N. Prognostic factors of renal cell carcinoma. J Urol. 2003. 169:821–827.15. Kim SW, Park WJ, Ha JS, Lee SJ, Lee JY, Lee CB, et al. Prognostic factors in renal cell carcinoma. Korean J Urol. 2002. 43:98–105.16. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982. 6:655–663.17. Medeiros LJ, Gelb AB, Weiss LM. Renal cell carcinoma. Prognostic significance of morphologic parameters in 121 cases. Cancer. 1988. 61:1639–1651.18. Skinner DG, Colvin RB, Vermillion CD, Pfister RC, Leadbetter WF. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer. 1971. 28:1165–1177.19. Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I. A heparin-binding angiogenic protein-basic fibroblast growth factor-is stored within basement membrane. Am J Pathol. 1988. 130:393–400.20. Jinzaki M, Tanimoto A, Mukai M, Ikeda E, Kobayashi S, Yuasa Y, et al. Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr. 2000. 24:835–842.21. Ruppert-Kohlmayr AJ, Uggowitzer M, Meissnitzer T, Ruppert G. Differentiation of renal clear cell carcinoma and renal papillary carcinoma using quantitative CT enhancement parameters. AJR Am J Roentgenol. 2004. 183:1387–1391.22. Chang SG, Jeon SH, Lee SJ, Choi JM, Kim YW. Clinical significance of urinary vascular endothelial factor and microvessel density in patients with renal cell carcinoma. Urology. 2001. 58:904–908.23. Delahunt B, Bethwaite PB, Thornton A. Prognostic significance of microscopic vascularity for clear cell renal cell carcinoma. Br J Urol. 1997. 80:401–404.24. Köhler HH, Barth PJ, Siebel A, Gerharz EW, Bittinger A. Quantitative assessment of vascular surface density in renal cell carcinomas. Br J Urol. 1996. 77:650–654.25. Folkmn J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995. 333:1757–1763.26. Peters W, Teixeira M, Intaglietta M, Gross JF. Microcirculatory studies in rat mammary carcinoma. I. Transparent chamber method, development of microvasculature, and pressures in tumor vessels. J Natl Cancer Inst. 1980. 65:631–642.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on the comparision of various imaging methods for the staging of renal cell carcinoma
- Correlation of CT Findings and Pathologic Nuclear Grading in Renal Cell Carcinoma
- Comparison of Ultrasonography, Computed Tomography and Excretory Urography in Staging of Renal Cell Carcinoma
- Correlation between Nuclear Grades and the Numbers of AgNORs and PCNA Labeling Indices in Renal Cell Carcinoma
- A Pathologic Study of Renal Cell Carcinoma: Correlation between clinical and morphologic parameters and prognosis