Korean J Urol.
2010 Jan;51(1):54-59.
Phosphaturia as a Promising Predictor of Recurrent Stone Formation in Patients with Urolithiasis
- Affiliations
-
- 1Department of Urology, College of Medicine, Chungbuk National University, Cheongju, Korea. urokyj@cbnu.ac.kr
Abstract
- PURPOSE
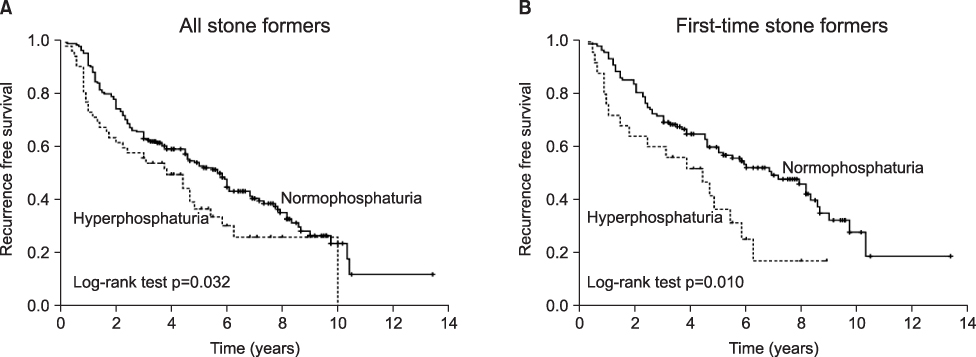
Recent studies have suggested that renal phosphate leakage and the associated phosphaturia are significant underlying causes of calcium urolithiasis. The aims of this study were to assess whether phosphaturia relates to urinary metabolic abnormalities and recurrent stone formation. MATERIALS AND METHODS: A database of patient histories and urine chemistries was analyzed for 1,068 consecutive stone formers (SFs) and 106 normal controls. Urine values for phosphaturia that were higher than 95% of the normal control values were defined as indicating hyperphosphaturia, and the effect of phosphaturia on urinary metabolites and stone recurrence was determined. Of these patients, 247 patients (23.1%) who had been followed up for more than 36 months or had a recurrence of stones during follow-up (median, 46.0 months; range, 5-151) were included in the analyses for stone recurrence. RESULTS: Of the SFs, 19.9% (212/1,068) had increased urinary phosphate excretion. SFs with hyperphosphaturia had a greater urinary volume and higher levels of calcium, uric acid, oxalate, and citrate than did SFs with normophosphaturia. A multivariate Cox regression model, stratified by stone episodes, revealed that hyperphosphaturia was an independent predictor of recurrent stone formation in first-time SFs (hazard ratio [HR]: 2.122; 95% confidence interval [CI]: 1.100-4.097; p=0.025). No association was detected between hyperphosphaturia and recurrent stone formation in recurrent SFs. Kaplan-Meier curves showed identical results. CONCLUSIONS: This study demonstrates that hyperphosphaturia is closely associated with urinary metabolic abnormalities. Furthermore, hyperphosphaturia is a significant risk factor for stone recurrence in first-time SFs.
Keyword
MeSH Terms
Figure
Reference
-
1. Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 2003. 63:1817–1823.2. Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol. 2003. 14:1082–1095.3. Lifshitz DA, Shalhav AL, Lingeman JE, Evan AP. Metabolic evaluation of stone disease patients: a practical approach. J Endourol. 1999. 13:669–678.4. Moe OW, Bonny O. Genetic hypercalciuria. J Am Soc Nephrol. 2005. 16:729–745.5. Kim MS, Moon YT. The relationship between obesity and the risk factors of urolithiasis. Korean J Urol. 2007. 48:505–511.6. Kim TH, Lee SY, Chung WH, Oh SY, Moon YT, Kim KD, et al. Eta-1/osteopontin genetic polymorphism is associated with urolithiasis in Koreans. Korean J Urol. 2008. 49:55–59.7. Kwon OJ, Ahn SH. Comparison of the lithogenic risk factors for first time and recurrent stone-formers. Korean J Urol. 2006. 47:1093–1098.8. Prie D, Ravery V, Boccon-Gibod L, Friedlander G. Frequency of renal phosphate leak among patients with calcium nephrolithiasis. Kidney Int. 2001. 60:272–276.9. Kim YJ, Kim TH, Yun SJ, Kim ME, Kim WJ, Lee SC. Renal phosphate control as a reliable predictive factor of stone recurrence. J Urol. 2009. 181:2566–2572.10. Karim Z, Gerard B, Bakouh N, Alili R, Leroy C, Beck L, et al. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med. 2008. 359:1128–1135.11. Levi M, Bruesegem S. Renal phosphate-transporter regulatory proteins and nephrolithiasis. N Engl J Med. 2008. 359:1171–1173.12. Williams CP, Child DF, Hudson PR, Soysa LD, Davies GK, Davies MG, et al. Inappropriate phosphate excretion in idiopathic hypercalciuria: the key to a common cause and future treatment? J Clin Pathol. 1996. 49:881–888.13. Tieder M, Modai D, Shaked U, Samuel R, Arie R, Halabe A, et al. "Idiopathic" hypercalciuria and hereditary hypophosphatemic rickets. Two phenotypical expressions of a common genetic defect. N Engl J Med. 1987. 316:125–129.14. Jo SW, Lee SC, Kim WJ. Predicting factors for recurrence in first-time stone formers. Korean J Urol. 2007. 48:176–182.15. Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006. 78:179–192.16. Prie D, Huart V, Bakouh N, Planelles G, Dellis O, Gerard B, et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002. 347:983–991.17. Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998. 95:5372–5377.18. Bushinsky DA. Nephrolithiasis: site of the initial solid phase. J Clin Invest. 2003. 111:602–605.19. Aihara K, Byer KJ, Khan SR. Calcium phosphate-induced renal epithelial injury and stone formation: involvement of reactive oxygen species. Kidney Int. 2003. 64:1283–1291.20. Murer H, Hernando N, Forster I, Biber J. Regulation of Na/Pi transporter in the proximal tubule. Annu Rev Physiol. 2003. 65:531–542.21. Weinman EJ, Mohanlal V, Stoycheff N, Wang F, Steplock D, Shenolikar S, et al. Longitudinal study of urinary excretion of phosphate, calcium, and uric acid in mutant NHERF-1 null mice. Am J Physiol Renal Physiol. 2006. 290:F838–F843.22. Cunningham R, Brazie M, Kanumuru S, E X, Biswas R, Wang F, et al. Sodium-hydrogen exchanger regulatory factor-1 interacts with mouse urate transporter 1 to regulate renal proximal tubule uric acid transport. J Am Soc Nephrol. 2007. 18:1419–1425.23. Hernando N, Deliot N, Gisler SM, Lederer E, Weinman EJ, Biber J, et al. PDZ-domain interactions and apical expression of type IIa Na/P(i) cotransporters. Proc Natl Acad Sci U S A. 2002. 99:11957–11962.24. Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci U S A. 2002. 99:11470–11475.25. Oh SY, Moon YT. Comparison of metabolic risk factors in patients with first-time and recurrent stone formations. Korean J Urol. 2004. 45:551–556.26. Hosseini MM, Eshraghian A, Dehghanian I, Irani D, Amini M. Metabolic abnormalities in patients with nephrolithiasis: comparison of first-episode with recurrent cases in Southern Iran. Int Urol Nephrol. 2009. Epub ahead of print.27. Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006. 367:333–344.28. Nishida Y, Ito K. Decreased renal phosphate threshold in patients with gout. Nephron. 1992. 62:142–144.29. Schwille PO, Schmiedl A, Herrmann U, Wipplinger J. Postprandial hyperinsulinaemia, insulin resistance and inappropriately high phosphaturia are features of younger males with idiopathic calcium urolithiasis: attenuation by ascorbic acid supplementation of a test meal. Urol Res. 1997. 25:49–58.30. Bushinsky DA, Asplin JR. Thiazides reduce brushite, but not calcium oxalate, supersaturation, and stone formation in genetic hypercalciuric stone-forming rats. J Am Soc Nephrol. 2005. 16:417–424.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Review of Pediatric Urolithiasis: Etiology and Treatment
- A Statistical Study of Recurrent Urolithiasis Preliminary Report
- Comparison of Metabolic Risk Factors in Patients with First-time and Recurrent Stone Formations
- Metabolic Abnormalities and the Risk for Recurrence in Obese Patients with Urolithiasis
- Risk factors for urinary stone