Obstet Gynecol Sci.
2014 Nov;57(6):539-543. 10.5468/ogs.2014.57.6.539.
Neoadjuvant and postoperative chemotherapy with paclitaxel plus cisplatin for the treatment of FIGO stage IB cervical cancer in pregnancy
- Affiliations
-
- 1Gynecologic Cancer Center, Department of Obstetrics and Gynecology, Ajou University School of Medicine, Suwon, Korea. hsryu@ajou.ac.kr
- 2Department of Radiology, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2314024
- DOI: http://doi.org/10.5468/ogs.2014.57.6.539
Abstract
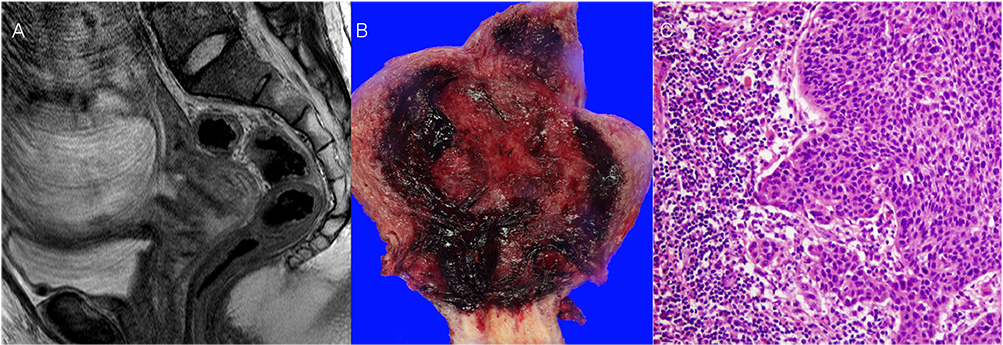
- Cervical cancer is one of the most common malignancy diagnosed during pregnancy. The experience of the use of neoadjuvant chemotherapy (NACT) with paclitaxel plus cisplatin during pregnancy is limited. Three pregnant women with International Federation of Gynecology and Obstetrics (FIGO) stage IB cervical cancer received NACT with paclitaxel plus cisplatin until fetal lung maturity, and then underwent cesarean delivery and radical hysterectomy. Two of our patients had intermediate pathologic risk factors, and received adjuvant chemotherapy with the same regimen used in NACT. All patients did not have any evidence of disease recurrence for follow-up of 3, 4, and 8 years, respectively. NACT with paclitaxel plus cisplatin followed by radical hysterectomy and adjuvant chemotherapy could be considered as one of feasible alternatives to primary radical surgery or concurrent chemoradiation therapy with the termination of pregnancy in pregnant women with FIGO stage IB cervical cancer who have two or more intermediate pathologic-risk factors.
Keyword
MeSH Terms
Figure
Reference
-
1. Pavlidis NA. Coexistence of pregnancy and malignancy. Oncologist. 2002; 7:279–287.2. Hunter MI, Tewari K, Monk BJ. Cervical neoplasia in pregnancy. Part 2: current treatment of invasive disease. Am J Obstet Gynecol. 2008; 199:10–18.3. Rose PG, Blessing JA, Gershenson DM, McGehee R. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 1999; 17:2676–2680.4. Palaia I, Pernice M, Graziano M, Bellati F, Panici PB. Neoadjuvant chemotherapy plus radical surgery in locally advanced cervical cancer during pregnancy: a case report. Am J Obstet Gynecol. 2007; 197:e5–e6.5. Chun KC, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Neoadjuvant chemotherapy with paclitaxel plus platinum followed by radical surgery in early cervical cancer during pregnancy: three case reports. Jpn J Clin Oncol. 2010; 40:694–698.6. Li J, Wang LJ, Zhang BZ, Peng YP, Lin ZQ. Neoadjuvant chemotherapy with paclitaxel plus platinum for invasive cervical cancer in pregnancy: two case report and literature review. Arch Gynecol Obstet. 2011; 284:779–783.7. Fruscio R, Villa A, Chiari S, Vergani P, Ceppi L, Dell'Orto F, et al. Delivery delay with neoadjuvant chemotherapy for cervical cancer patients during pregnancy: a series of nine cases and literature review. Gynecol Oncol. 2012; 126:192–197.8. Brewer M, Kueck A, Runowicz CD. Chemotherapy in pregnancy. Clin Obstet Gynecol. 2011; 54:602–618.9. Morice P, Uzan C, Gouy S, Verschraegen C, Haie-Meder C. Gynaecological cancers in pregnancy. Lancet. 2012; 379:558–569.10. Karam A, Feldman N, Holschneider CH. Neoadjuvant cisplatin and radical cesarean hysterectomy for cervical cancer in pregnancy. Nat Clin Pract Oncol. 2007; 4:375–380.11. Lai CH, Hsueh S, Chang TC, Tseng CJ, Huang KG, Chou HH, et al. Prognostic factors in patients with bulky stage IB or IIA cervical carcinoma undergoing neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol. 1997; 64:456–462.12. Rabaiotti E, Sigismondi C, Montoli S, Mangili G, Candiani M, Vigano R. Management of locally advanced cervical cancer in pregnancy: a case report. Tumori. 2010; 96:623–626.13. Zemlickis D, Klein J, Moselhy G, Koren G. Cisplatin protein binding in pregnancy and the neonatal period. Med Pediatr Oncol. 1994; 23:476–479.14. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999; 73:177–183.15. Takeshima N, Umayahara K, Fujiwara K, Hirai Y, Takizawa K, Hasumi K. Treatment results of adjuvant chemotherapy after radical hysterectomy for intermediate- and high-risk stage IB-IIA cervical cancer. Gynecol Oncol. 2006; 103:618–622.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phase II Study of CP (Cisplatin/Paclitaxel) as Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Carcinoma
- Successful Pregnancy after Paclitaxel-Cisplatin Chemotherapy in Invasive Squamous Cell Carcinoma of Cervix During Pregnancy: A Case Report and Review of the Literature
- Phase II trial of Neoadjuvant Paclitaxel and Cisplatin in Carcinoma of the Uterine Cervix
- Neoadjuvant chemotherapy in cervical carcinoma during pregnancy: Case report
- Phase II Study of Cisplatin, Ifosfamide . Paclitaxel (CIP) as Neoadjuvant Chemotherapy in Patients with Locally Advanced Cervical Carcinoma