Obstet Gynecol Sci.
2014 Nov;57(6):464-470. 10.5468/ogs.2014.57.6.464.
Protective effects of green tea polyphenol against cisplatin-induced nephrotoxicity in rats
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Chosun University College of Medicine, Gwangju, Korea. sjhan@chosun.ac.kr
- 2Department of Biochemistry and Molecular Biology, Chosun University College of Medicine, Gwangju, Korea.
- KMID: 2314012
- DOI: http://doi.org/10.5468/ogs.2014.57.6.464
Abstract
OBJECTIVE
This study is to compare the effects of green tea polyphenol (GTP) pre-treatment with those of GTP post-treatment on cisplatin (CP)-induced nephrotoxicity in rat.
METHODS
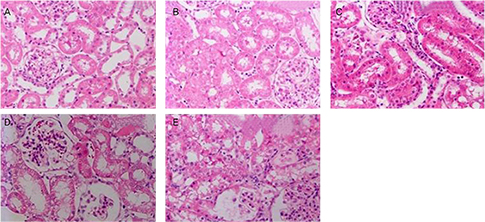
Male Sprague-Dawley rats were randomly divided into six groups. Animals in the control group received 0.9% saline (intraperitoneal); animals in the GTP group received 0.9% saline and GTP (0.2% GTP as their sole source of drinking water); the CP group received only CP (7 mg/kg, intraperitoneal); the CP+preGTP group received GTP from two days before CP to four days after CP and the CP+postGTP group received GTP for four days after CP. CP-induced renal toxicity was evaluated by plasma creatinine and blood urea nitrogen (BUN) concentrations; kidney tissue gamma-glutamyl transpeptidase (GGT) and alkaline phosphatase (AP) activities and histopathological examinations.
RESULTS
High serume creatinine and BUN concentrations were observed in CP treated rats. The GGT and AP activites were lower in kidney of CP treated rats compared to control rats. In addition, treatment with CP resulted in development of a marked tubular necrosis, and tubular dilation in kidney of rats. Pretreatment with GTP resulted in markedly reduced elevation of serum creatinine and BUN amounts and changes of GGT and AP activity in kidney induced by CP. CP-induced histopathological changes, including tubular necrosis and dilation, were ameliorated in GTP pre-treated rats, compared to CP alone or GTP post-treated rats.
CONCLUSION
These results demonstrate that GTP might have some protective effect against CP-induced nephrotoxicity in rat, and GTP pre-treatment was more effective than GTP post-treatment on reduction of CP-induced renal dysfunction.
Keyword
MeSH Terms
Figure
Reference
-
1. Hill JM, Loeb E, MacLellan A, Hill NO, Khan A, King JJ. Clinical studies of platinum coordination compounds in the treatment of various malignant diseases. Cancer Chemother Rep. 1975; 59:647–659.2. Hill JM, Speer RJ. Organo-platinum complexes as antitumor agents (review). Anticancer Res. 1982; 2:173–186.3. Matsuyama R, Reddy S, Smith TJ. Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol. 2006; 24:3490–3496.4. Bartelink H, Schellens JH, Verheij M. The combined use of radiotherapy and chemotherapy in the treatment of solid tumours. Eur J Cancer. 2002; 38:216–222.5. Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003; 23:460–464.6. Dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol. 2012; 86:1233–1250.7. Sadzuka Y, Shoji T, Takino Y. Effect of cisplatin on the activities of enzymes which protect against lipid peroxidation. Biochem Pharmacol. 1992; 43:1872–1875.8. Appenroth D, Frob S, Kersten L, Splinter FK, Winnefeld K. Protective effects of vitamin E and C on cisplatin nephrotoxicity in developing rats. Arch Toxicol. 1997; 71:677–683.9. Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004; 195:221–230.10. Atessahin A, Ceribasi AO, Yuce A, Bulmus O, Cikim G. Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin Pharmacol Toxicol. 2007; 100:121–126.11. Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010; 2:1231–1246.12. Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010; 501:65–72.13. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003; 43:89–143.14. Yang CS, Wang X. Green tea and cancer prevention. Nutr Cancer. 2010; 62:931–937.15. El-Mowafy AM, Salem HA, Al-Gayyar MM, El-Mesery ME, El-Azab MF. Evaluation of renal protective effects of the green-tea (EGCG) and red grape resveratrol: role of oxidative stress and inflammatory cytokines. Nat Prod Res. 2011; 25:850–856.16. Sahin K, Tuzcu M, Gencoglu H, Dogukan A, Timurkan M, Sahin N, et al. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010; 87:240–245.17. Khan SA, Priyamvada S, Khan W, Khan S, Farooq N, Yusufi AN. Studies on the protective effect of green tea against cisplatin induced nephrotoxicity. Pharmacol Res. 2009; 60:382–391.18. Tate SS, Meister A. Gamma-glutamyl transpeptidase from kidney. Methods Enzymol. 1985; 113:400–419.19. Tenenhouse HS, Scriver CR, Vizel EJ. Alkaline phosphatase activity does not mediate phosphate transport in the renal-cortical brush-border membrane. Biochem J. 1980; 190:473–476.20. Fatima S, Arivarasu NA, Mahmood R. Vitamin C attenuates cisplatin-induced alterations in renal brush border membrane enzymes and phosphate transport. Hum Exp Toxicol. 2007; 26:419–426.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Antiproteinuric Effects of Green Tea Polyphenol on Cyclosporine A-Induced Acute Renal Injury in Mice
- The Effect of Green Tea Extract on Cisplatin in Cervical Cancer Cell Lines
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts
- Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies
- Erythropoietin (EPO) Attenuates Renal Injury in an Experimental Model of Cisplatin-induced Nephrotoxicity in Rats