Obstet Gynecol Sci.
2014 Mar;57(2):109-114.
Comparison of oxidative stress markers in umbilical cord blood after vaginal and cesarean delivery
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Chonnam National University Medical School, Gwangju, Korea. kimyh@jnu.ac.kr
- 2Department of Obstetrics and Gynecology, Chonnam National University Research Institute of Medical Sciences, Gwangju, Korea.
Abstract
OBJECTIVE
The purpose of our study was to investigate the effect of the mode of delivery on the oxidant and antioxidant system in umbilical cord blood.
METHODS
We performed gas analysis of umbilical venous blood and umbilical arterial blood immediately after delivery in 38 women; eighteen women had a vaginal delivery while 20 women delivered via cesarean section at over 37 weeks gestation. We examined lipid peroxide concentration by thiobarbituric acid reaction, protein carbonyl content by 2,4-dinitrophenylhydrazine reaction, and total antioxidant capacity by oxygen radical absorbance capacity assay.
RESULTS
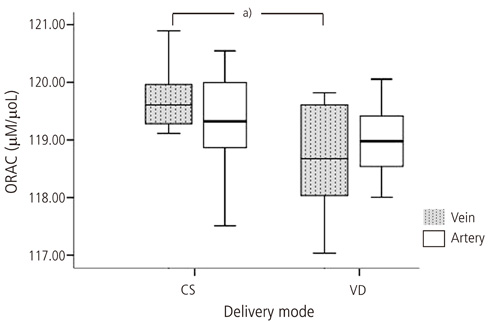
Lipid peroxide levels in umbilical venous blood were significantly higher in patients delivering by planned cesarean section (1.81 +/- 0.06 nmol/mg protein) than those with vaginal delivery (1.24 +/- 0.05 nmol/mg protein) (P < 0.05). Antioxidant capacity in umbilical venous blood was significantly higher in patients delivering by planned cesarean section (119.70 +/- 0.13 microM/microL) than those with a vaginal delivery (118.70 +/- 0.29 microM/microL) (P < 0.05). There was no significant difference in the carbonyl content of umbilical venous blood or in the lipid peroxide, carbonyl content, and total antioxidant capacity of umbilical arterial blood.
CONCLUSION
Lipid peroxidation levels and antioxidant capacity in umbilical venous blood were higher in patients delivering by planned cesarean section than those with a vaginal delivery. Therefore, we propose that both the mother and neonate are exposed to higher oxidative stress during cesarean section delivery.
MeSH Terms
Figure
Reference
-
1. Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994; 344:721–724.2. Fridovich I. Oxygen toxicity: a radical explanation. J Exp Biol. 1998; 201(Pt 8):1203–1209.3. Yaacobi N, Ohel G, Hochman A. Reactive oxygen species in the process of labor. Arch Gynecol Obstet. 1999; 263:23–24.4. Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol (Oxf). 2002; 57:609–613.5. Walsh SW, Wang Y. Secretion of lipid peroxides by the human placenta. Am J Obstet Gynecol. 1993; 169:1462–1466.6. Clerici G, Slavescu C, Fiengo S, Kanninen TT, Romanelli M, Biondi R, et al. Oxidative stress in pathological pregnancies. J Obstet Gynaecol. 2012; 32:124–127.7. Mondal N, Bhat BV, Banupriya C, Koner BC. Oxidative stress in perinatal asphyxia in relation to outcome. Indian J Pediatr. 2010; 77:515–517.8. Akisu M, Kullahcioglu Girgin F, Baka M, Husseyinov A, Kultursay N. The role of recombinant human erythropoietin in lipid peroxidation and platelet-activating factor generation in a rat model of necrotizing enterocolitis. Eur J Pediatr Surg. 2001; 11:167–172.9. Yiğit S, Yurdakok M, Kilinc K, Oran O, Erdem G, Tekinalp G. Serum malondialdehyde concentration as a measure of oxygen free radical damage in preterm infants. Turk J Pediatr. 1998; 40:177–183.10. Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res. 2009; 66:121–127.11. Siddiqui IA, Jaleel A, Tamimi W, Al Kadri HM. Role of oxidative stress in the pathogenesis of preeclampsia. Arch Gynecol Obstet. 2010; 282:469–474.12. Aydemir O, Akar M, Uras N, Eras Z, Erdeve O, Oguz SS, et al. Total antioxidant capacity and total oxidant status in perinatal asphyxia in relation to neurological outcome. Neuropediatrics. 2011; 42:222–226.13. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.14. Oliver CN, Ahn BW, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J Biol Chem. 1987; 262:5488–5491.15. Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med. 1993; 14:303–311.16. Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, LLeonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013; 12:376–390.17. Giles GI, Tasker KM, Jacob C. Hypothesis: the role of reactive sulfur species in oxidative stress. Free Radic Biol Med. 2001; 31:1279–1283.18. Simic MG, Bergtold DS, Karam LR. Generation of oxy radicals in biosystems. Mutat Res. 1989; 214:3–12.19. Perry G, Friedland RP, Petot GJ, Nunomura A, Castellani RJ, Kubat Z, et al. Alzheimer as a disease of metabolic demand:benefits of physical and brain exercise. In : Radak Z, editor. Exercise and diseases: prevention through training. Oxford: Meyer & Meyer Sport;2005. p. 7–16.20. Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005; 6:71–75.21. Yu BP, Chung HY. Adaptive mechanisms to oxidative stress during aging. Mech Ageing Dev. 2006; 127:436–443.22. Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006; 7:278–294.23. Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005; 16:129–137.24. Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004; 122:369–382.25. Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004; 11:342–352.26. Haga P, Lunde G. Selenium and vitamin E in cord blood from preterm and full term infants. Acta Paediatr Scand. 1978; 67:735–739.27. Gopinathan V, Miller NJ, Milner AD, Rice-Evans CA. Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett. 1994; 349:197–200.28. Hara K, Yamashita S, Fujisawa A, Ishiwa S, Ogawa T, Yamamoto Y. Oxidative stress in newborn infants with and without asphyxia as measured by plasma antioxidants and free fatty acids. Biochem Biophys Res Commun. 1999; 257:244–248.29. Aydemir C, Dilli D, Uras N, Ulu HO, Oguz SS, Erdeve O, et al. Total oxidant status and oxidative stress are increased in infants with necrotizing enterocolitis. J Pediatr Surg. 2011; 46:2096–2100.30. Russell AP, Hesselink MK, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J. 2005; 19:986–988.31. Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008; 7:34–42.32. Mutlu B, Aksoy N, Cakir H, Celik H, Erel O. The effects of the mode of delivery on oxidative-antioxidative balance. J Matern Fetal Neonatal Med. 2011; 24:1367–1370.33. Khaw KS, Wang CC, Ngan Kee WD, Pang CP, Rogers MS. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002; 88:18–23.34. Khaw KS, Wang CC, Ngan Kee WD, Tam WH, Ng FF, Critchley LA, et al. Supplementary oxygen for emergency Caesarean section under regional anaesthesia. Br J Anaesth. 2009; 102:90–96.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Delivery Mode on Fetal Stress Hormones and Acid-Base Status
- Umbilical Cord Arterial Blood pH Analysis at Term Pregnancy
- Vaginal Birth after Cesarean Delivery : Development of a Scoring System for Predicting Success Rate
- A Study Female of Fecal Incontinence: Effects of Parity & Delivery method
- Analysis of Affecting Factors for Cortisol Level in Cord Blood