Nutr Res Pract.
2016 Apr;10(2):131-138. 10.4162/nrp.2016.10.2.131.
Bioconverted Jeju Hallabong tangor (Citrus kiyomi × ponkan) peel extracts by cytolase enhance antioxidant and anti-inflammatory capacity in RAW 264.7 cells
- Affiliations
-
- 1Department of Food and Nutrition, College of Natural Sciences, Myongji University, 116 Myongji-ro, Cheoin-gu, Yongin, Gyeonggi-do 17058, Korea. jhwang@mju.ac.kr
- 2BK Bio Co. Ltd., Sangdaewon-dong, Jungwon-gu, Seongnam, Gyeonggi-do 13229, Korea.
- 3Department of Food and Nutrition, Soongeui Women's College, Seoul 04628, Korea.
- 4Department of Food Science and Nutrition, Yongin University, Yongin, Gyeonggi-do 17092, Korea.
- KMID: 2313909
- DOI: http://doi.org/10.4162/nrp.2016.10.2.131
Abstract
- BACKGROUND/OBJECTIVES
Citrus and its peels have been used in Asian folk medicine due to abundant flavonoids and usage of citrus peels, which are byproducts from juice and/or jam processing, may be a good strategy. Therefore, the aim of this study was to examine antioxidant and anti-inflammatory effects of bioconversion of Jeju Hallabong tangor (Citrus kiyomi × ponkan; CKP) peels with cytolase (CKP-C) in RAW 264.7 cells.
MATERIALS/METHODS
Glycosides of CKP were converted into aglycosides with cytolase treatment. RAW 264.7 cells were pre-treated with 0, 100, or 200 µg/ml of citrus peel extracts for 4 h, followed by stimulation with 1 µg/ml lipopolysaccharide (LPS) for 8 h. Cell viability, DPPH radical scavenging activity, nitric oxide (NO), and prostagladin E2 (PGE2) production were examined. Real time-PCR and western immunoblotting assay were performed for detection of mRNA and/or protein expression of pro-inflammatory mediators and cytokines, respectively.
RESULTS
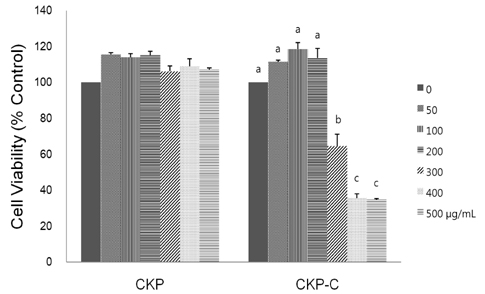
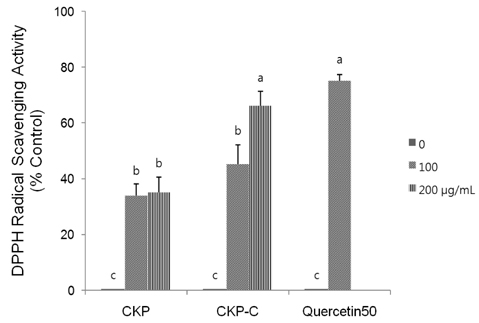
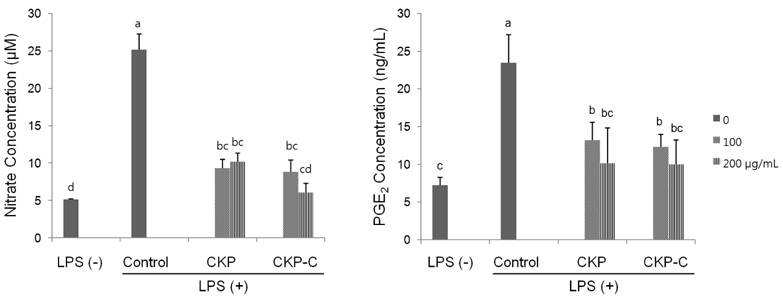
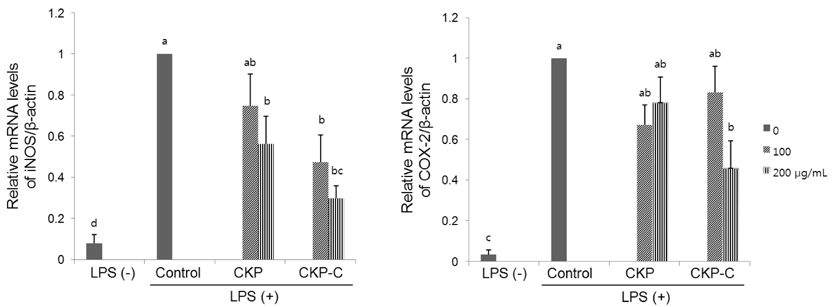
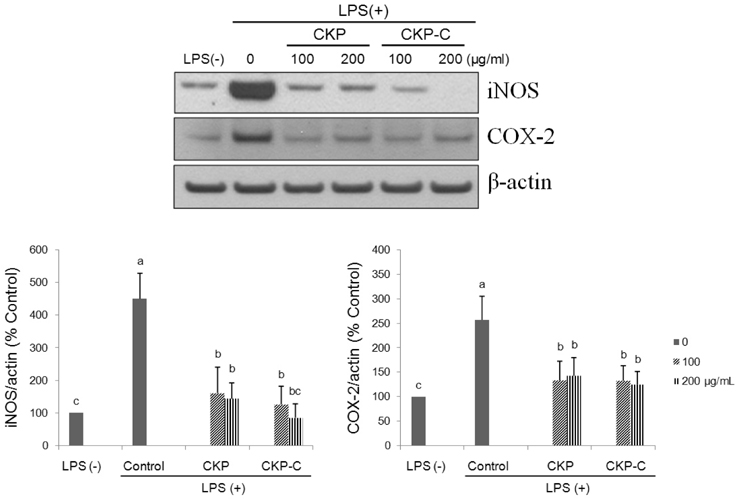
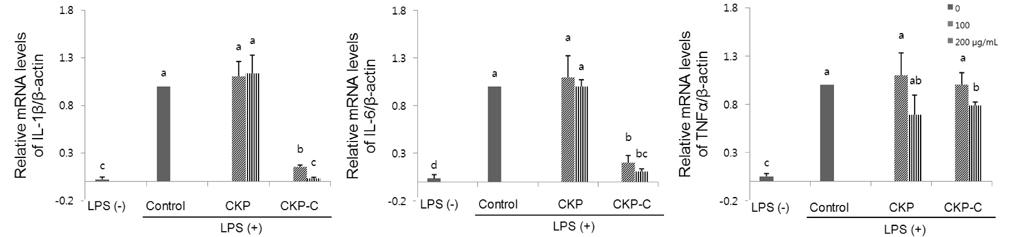
HPLC analysis showed that treatment of CKP with cytolase resulted in decreased flavanone rutinoside forms (narirutin and hesperidin) and increased flavanone aglycoside forms (naringenin and hesperetin). DPPH scavenging activities were observed in a dose-dependent manner for all of the citrus peel extracts and CKP-C was more potent than intact CKP. All of the citrus peel extracts decreased NO production by inducible nitric oxide synthase (iNOS) activity and PGE2 production by COX-2. Higher dose of CKP and all CKP-C groups significantly decreased mRNA and protein expression of LPS-stimulated iNOS. Only 200 µg/ml of CKP-C markedly decreased mRNA and protein expression of cyclooxygenase-2 in LPS-stimulated RAW 264.7 cells. Both 100 and 200 µg/ml of CKP-C notably inhibited mRNA levels of interleukin-1β (IL-1β) and IL-6, whereas 200 µg/ml CKP-C significantly inhibited mRNA levels of TNF-α.
CONCLUSIONS
This result suggests that bioconversion of citrus peels with cytolase may enrich aglycoside flavanones of citrus peels and provide more potent functional food materials for prevention of chronic diseases attributable to oxidation and inflammation by increasing radical scavenging activity and suppressing pro-inflammatory mediators and cytokines.
MeSH Terms
-
Asian Continental Ancestry Group
Blotting, Western
Cell Survival
Chromatography, High Pressure Liquid
Chronic Disease
Citrus
Cyclooxygenase 2
Cytokines
Dinoprostone
Flavanones
Flavonoids
Functional Food
Glycosides
Humans
Inflammation
Interleukin-6
Medicine, Traditional
Nitric Oxide
Nitric Oxide Synthase Type II
RNA, Messenger
Cyclooxygenase 2
Cytokines
Dinoprostone
Flavanones
Flavonoids
Glycosides
Interleukin-6
Nitric Oxide
Nitric Oxide Synthase Type II
RNA, Messenger
Figure
Reference
-
1. Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000; 6:347–373.
Article2. Tracey KJ. The inflammatory reflex. Nature. 2002; 420:853–859.
Article3. Fan GW, Zhang Y, Jiang X, Zhu Y, Wang B, Su L, Cao W, Zhang H, Gao X. Anti-inflammatory activity of baicalein in LPS-stimulated RAW 264.7 macrophages via estrogen receptor and NF-κB-dependent pathways. Inflammation. 2013; 36:1584–1591.
Article4. Hu W, Yang X, Zhe C, Zhang Q, Sun L, Cao K. Puerarin inhibits iNOS, COX-2 and CRP expression via suppression of NF-κB activation in LPS-induced RAW 264.7 macrophage cells. Pharmacol Rep. 2011; 63:781–789.
Article5. Seo JE, Lim HJ, Chang YH, Park HR, Han BK, Jeong JK, Choi KS, Park SB, Choi HJ, Hwang JA. Effects of Jeju Citrus unshiu peel extracts before and after bioconversion with cytolase on anti-inflammatory activity in RAW 264.7 Cells. J Korean Soc Food Sci Nutr. 2015; 44:331–337.
Article6. Lee C, Oh H, Han S, Lim S. Effects of hot air and freeze drying methods on physicochemical properties of citrus 'Hallabong' powders. Food Sci Biotechnol. 2012; 21:1633–1639.
Article7. Treutter D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol (Stuttg). 2005; 7:581–591.
Article8. Walle T. Absorption and metabolism of flavonoids. Free Radic Biol Med. 2004; 36:829–837.
Article9. Bocco A, Cuvelier ME, Richard H, Berset C. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J Agric Food Chem. 1998; 46:2123–2129.
Article10. Higashi-Okai K, Kamimoto K, Yoshioka A, Okai Y. Potent suppressive activity of fresh and dried peels from Satsuma mandarin Citrus unshiu (Marcorv.) on hydroperoxide generation from oxidized linoleic acid. Phytother Res. 2002; 16:781–784.
Article11. Suzuki M, Sasaki K, Yoshizaki F, Oguchi K, Fujisawa M, Cyong JC. Anti-hepatitis C virus effect of Citrus unshiu peel and its active ingredient nobiletin. Am J Chin Med. 2005; 33:87–94.
Article12. Jo CU, Park BJ, Chung SH, Kim CB, Cha BS, Byun MW. Antibacterial and anti-fungal activity of citrus (Citrus unshiu) essential oil extracted from peel by-products. Food Sci Biotechnol. 2004; 13:384–386.13. Lee S, Ra J, Song JY, Gwak C, Kwon HJ, Yim SV, Hong SP, Kim J, Lee KH, Cho JJ, Park YS, Park CS, Ahn HJ. Extracts from Citrus unshiu promote immune-mediated inhibition of tumor growth in a murine renal cell carcinoma model. J Ethnopharmacol. 2011; 133:973–979.
Article14. Peterson J, Dwyer J. Flavonoids: dietary occurrence and biochemical activity. Nutr Res. 1998; 18:1995–2018.
Article15. Tripoli E, La Guardia M, Giammanco S, Di Majo D, Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007; 104:466–479.
Article16. Fuhr U, Kummert AL. The fate of naringin in humans: a key to grapefruit juice-drug interactions? Clin Pharmacol Ther. 1995; 58:365–373.
Article17. Seo JY, Lee JH, Kim NW, Her E, Chang SH, Ko NY, Yoo YH, Kim JW, Seo DW, Han JW, Kim YM, Choi WS. Effect of a fermented ginseng extract, BST204, on the expression of cyclooxygenase-2 in murine macrophages. Int Immunopharmacol. 2005; 5:929–936.
Article18. Yang HL, Chen SC, Senthil Kumar KJ, Yu KN, Lee Chao PD, Tsai SY, Hou YC, Hseu YC. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: an ex vivo approach. J Agric Food Chem. 2012; 60:522–532.
Article19. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995; 28:25–30.
Article20. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005; 81:230S–242S.
Article21. Marín L, Miguélez EM, Villar CJ, Lombó F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. BioMed Res Int. 2015; 2015:905215.
Article22. Bae EA, Han MJ, Kim DH. In vitro anti-Helicobacter pylori activity of some flavonoids and their metabolites. Planta Med. 1999; 65:442–443.
Article23. Kim HK, Jeon WK, Ko BS. Flavanone glycosides from Citrus junos and their anti-influenza virus activity. Planta Med. 2001; 67:548–549.
Article24. Chun SS, Vattem DA, Lin YT, Shetty K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 2005; 40:809–816.
Article25. Chao CL, Weng CS, Chang NC, Lin JS, Kao ST, Ho FM. Naringenin more effectively inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in macrophages than in microglia. Nutr Res. 2010; 30:858–864.
Article26. Blancke F, Claeys MJ, Jorens P, Vermeiren G, Bosmans J, Wuyts FL, Vrints CJ. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediators Inflamm. 2005; 2005:385–389.
Article27. Kang K. Citrus peel extract inhibits LPS-induced cytokines secretion in macrophage. Int J Bio-Sci Bio-Tech. 2014; 6:11–20.
Article28. Kang SR, Han DY, Park KI, Park HS, Cho YB, Lee HJ, Lee WS, Ryu CH, Ha YL, Lee DH, Kim JA, Kim GS. Suppressive effect on lipopolysaccharide-induced proinflammatory mediators by Citrus aurantium L. in macrophage RAW 264.7 cells via NF-κB signal pathway. Evid Based Complement Alternat Med. 2011; 2011:248592.29. Jung KH, Ha E, Kim MJ, Won HJ, Zheng LT, Kim HK, Hong SJ, Chung JH, Yim SV. Suppressive effects of nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression by Citrus reticulata extract in RAW 264.7 macrophage cells. Food Chem Toxicol. 2007; 45:1545–1550.
Article30. Lim H, Yeo E, Song E, Chang YH, Han BK, Choi HJ, Hwang J. Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells. Nutr Res Pract. 2015; 10:599–605.31. Cho EJ, Li L, Yamabe N, Kim HY. Antioxidative effects of hesperidin and hesperetin under cellular system. CNU J Agric Sci. 2011; 38:717–722.32. Cho J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch Pharm Res. 2006; 29:699–706.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells
- Anti-inflammatory Effect of Mangosteen (Garcinia mangostana L.) Peel Extract and its Compounds in LPS-induced RAW264.7 Cells
- Anti-inflammatory and Anti-oxidant Effects of Sophora flavescens Root Extraction in Lipopolysaccharide-activated Raw 264.7 Cells
- Effects of Different Mandarin Formulations on Antioxidative Capacity and Oxidative DNA Damage in Fifteen-Month Aged Rats
- Inhibitory Effect of Ginsenosides Rh1 and Rg2 on Oxidative Stress in LPS-Stimulated RAW 264.7 Cells